Losartan Blocks Osteosarcoma-Elicited Monocyte Recruitment, and Combined With the Kinase Inhibitor Toceranib, Exerts Significant Clinical Benefit in Canine Metastatic Osteosarcoma
- PMID: 34580111
- PMCID: PMC8866227
- DOI: 10.1158/1078-0432.CCR-21-2105
Losartan Blocks Osteosarcoma-Elicited Monocyte Recruitment, and Combined With the Kinase Inhibitor Toceranib, Exerts Significant Clinical Benefit in Canine Metastatic Osteosarcoma
Abstract
Purpose: There is increasing recognition that progress in immuno-oncology could be accelerated by evaluating immune-based therapies in dogs with spontaneous cancers. Osteosarcoma (OS) is one tumor for which limited clinical benefit has been observed with the use of immune checkpoint inhibitors. We previously reported the angiotensin receptor blocker losartan suppressed metastasis in preclinical mouse models through blockade of CCL2-CCR2 monocyte recruitment. Here we leverage dogs with spontaneous OS to determine losartan's safety and pharmacokinetics associated with monocyte pharmacodynamic endpoints, and assess its antitumor activity, in combination with the kinase inhibitor toceranib.
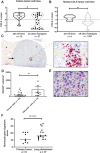
Patients and methods: CCL2 expression, monocyte infiltration, and monocyte recruitment by human and canine OS tumors and cell lines were assessed by gene expression, ELISA, and transwell migration assays. Safety and efficacy of losartan-toceranib therapy were evaluated in 28 dogs with lung metastatic OS. Losartan PK and monocyte PD responses were assessed in three dose cohorts of dogs by chemotaxis, plasma CCL2, and multiplex cytokine assays, and RNA-seq of losartan-treated human peripheral blood mononuclear cells.
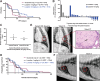
Results: Human and canine OS cells secrete CCL2 and elicit monocyte migration, which is inhibited by losartan. Losartan PK/PD studies in dogs revealed that a 10-fold-higher dose than typical antihypertensive dosing was required for blockade of monocyte migration. Treatment with high-dose losartan and toceranib was well-tolerated and induced a clinical benefit rate of 50% in dogs with lung metastases.
Conclusions: Losartan inhibits the CCL2-CCR2 axis, and in combination with toceranib, exerts significant biological activity in dogs with metastatic osteosarcoma, supporting evaluation of this drug combination in patients with pediatric osteosarcoma. See related commentary by Weiss et al., p. 571.
©2021 The Authors; Published by the American Association for Cancer Research.
Figures




Comment in
-
Truly Man's Best Friend: Canine Cancers Drive Drug Repurposing in Osteosarcoma.Clin Cancer Res. 2022 Feb 15;28(4):571-572. doi: 10.1158/1078-0432.CCR-21-3471. Clin Cancer Res. 2022. PMID: 34880110
References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

