Bardet-Biedl syndrome proteins modulate the release of bioactive extracellular vesicles
- PMID: 34580290
- PMCID: PMC8476602
- DOI: 10.1038/s41467-021-25929-1
Bardet-Biedl syndrome proteins modulate the release of bioactive extracellular vesicles
Abstract
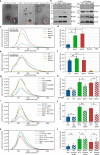
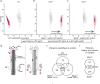
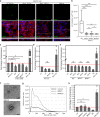
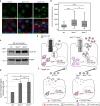
Primary cilia are microtubule based sensory organelles important for receiving and processing cellular signals. Recent studies have shown that cilia also release extracellular vesicles (EVs). Because EVs have been shown to exert various physiological functions, these findings have the potential to alter our understanding of how primary cilia regulate specific signalling pathways. So far the focus has been on lgEVs budding directly from the ciliary membrane. An association between cilia and MVB-derived smEVs has not yet been described. We show that ciliary mutant mammalian cells demonstrate increased secretion of small EVs (smEVs) and a change in EV composition. Characterisation of smEV cargo identified signalling molecules that are differentially loaded upon ciliary dysfunction. Furthermore, we show that these smEVs are biologically active and modulate the WNT response in recipient cells. These results provide us with insights into smEV-dependent ciliary signalling mechanisms which might underly ciliopathy disease pathogenesis.
© 2021. The Author(s).
Conflict of interest statement
António Domingues is currently an employee of Dewpoint Therapeutics. The remaining authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

