Evidence of possible SARS-CoV-2 vertical transmission according to World Health Organization criteria in asymptomatic pregnant women
- PMID: 34580942
- PMCID: PMC8661610
- DOI: 10.1002/uog.24787
Evidence of possible SARS-CoV-2 vertical transmission according to World Health Organization criteria in asymptomatic pregnant women
Abstract
Objective: Severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) vertical transmission has been investigated extensively. Recently, the World Health Organization (WHO) published strict criteria to classify the timing of mother-to-child transmission of SARS-CoV-2 into different categories. The aim of this study was to investigate the possibility of vertical transmission in asymptomatic SARS-CoV-2-positive women.
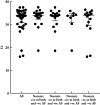
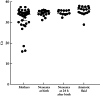
Methods: Pregnant women attending for delivery at a perinatology center in Mexico City, Mexico, who had a SARS-CoV-2-positive nasopharyngeal swab 24-48 h before delivery, were asymptomatic at the time of the test and had an obstetric indication for Cesarean section were eligible for inclusion in this study. Amniotic fluid was collected during Cesarean delivery, and neonatal oral and rectal swabs were collected at birth and at 24 h after birth. SARS-CoV-2 detection was carried out using real-time reverse-transcription polymerase chain reaction in all samples. Relevant medical information was retrieved from clinical records. The WHO criteria for classifying the timing of mother-to-child transmission of SARS-CoV-2 were applied to the study population.
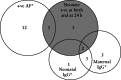
Results: Forty-two SARS-CoV-2-positive asymptomatic pregnant women fulfilled the inclusion criteria. Twenty-five (59%) women developed mild disease after discharge. Neonatal death occurred in three (7%) cases, of which one had a positive SARS-CoV-2 test at birth and none had coronavirus disease 2019-related symptoms. There were five (12%) cases with strong evidence of intrauterine transmission of SARS-CoV-2, according to the WHO criteria, as amniotic fluid samples and neonatal samples at birth and at 24 h after birth were positive for SARS-CoV-2. Our results also showed that 40-60% of infected neonates would have been undetected if only one swab (oral or rectal) was tested.
Conclusion: This study contributes evidence to reinforce the potential for vertical transmission of SARS-CoV-2 even in asymptomatic women and highlights the importance of testing more than one neonatal sample in order to increase the detection rate of SARS-CoV-2 in affected cases. © 2021 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Keywords: COVID-19; SARS-CoV-2; newborn testing; pregnancy; vertical transmission.
© 2021 The Authors. Ultrasound in Obstetrics & Gynecology published by John Wiley & Sons Ltd on behalf of International Society of Ultrasound in Obstetrics and Gynecology.
Figures


References
-
- Johns Hopkins University . Johns Hopkins University Coronavirus COVID‐19 Global Cases . https://coronavirus.jhu.edu/map.html.
-
- Mongula JE, Frenken MWE, van Lijnschoten G, Arents NLA, de Wit‐Zuurendonk LD, Schimmel‐de Kok APA, van Runnard Heimel PJ, Porath MM, Goossens SMTA. COVID‐19 during pregnancy: non‐reassuring fetal heart rate, placental pathology and coagulopathy. Ultrasound Obstet Gynecol 2020; 56: 773–776. - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

