Cooperative NHC/Photoredox Catalyzed Ring-Opening of Aryl Cyclopropanes to 1-Aroyloxylated-3-Acylated Alkanes
- PMID: 34580972
- PMCID: PMC9298441
- DOI: 10.1002/anie.202110304
Cooperative NHC/Photoredox Catalyzed Ring-Opening of Aryl Cyclopropanes to 1-Aroyloxylated-3-Acylated Alkanes
Abstract
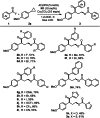
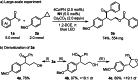
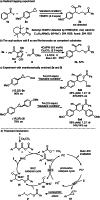
Cyclopropanes are an important class of building blocks in organic synthesis. Herein, a ring-opening/arylcarboxylation/acylation cascade reaction for the 1,3-difunctionalization of aryl cyclopropanes enabled by cooperative NHC and organophotoredox catalysis is reported. The cascade works on monosubstituted cyclopropanes that are in contrast to the heavily investigated donor-acceptor cyclopropanes more challenging to be difunctionalized. The key step is a radical/radical cross coupling of a benzylic radical generated in the photoredox catalysis cycle with a ketyl radical from the NHC catalysis cycle. The transformation features metal-free reaction conditions and tolerates a diverse range of functionalities.
Keywords: N-heterocyclic carbenes; cascade reactions; cyclopropanes; photoredox catalysis; reaction mechanisms.
© 2021 The Authors. Angewandte Chemie International Edition published by Wiley-VCH GmbH.
Conflict of interest statement
The authors declare no conflict of interest.
Figures



References
-
- For a book: see:
-
- Kulinkovich O. G., Cyclopropanes in Organic Synthesis, Wiley-VCH, Weinheim, 2015; For selected reviews, see:
-
- de Meijere A., Angew. Chem. Int. Ed. Engl. 1979, 18, 809;
- Angew. Chem. 1979, 91, 867;
-
- Wong H. N. C., Hon M. Y., Tse C. W., Yip Y. C., Tanko J., Hudlicky T., Chem. Rev. 1989, 89, 165;
-
- Rubin M., Rubina M., Gevorgyan V., Chem. Rev. 2007, 107, 3117; - PubMed
Grants and funding
LinkOut - more resources
Full Text Sources

