An open label randomized controlled trial of tamoxifen combined with amphotericin B and fluconazole for cryptococcal meningitis
- PMID: 34581270
- PMCID: PMC8547950
- DOI: 10.7554/eLife.68929
An open label randomized controlled trial of tamoxifen combined with amphotericin B and fluconazole for cryptococcal meningitis
Abstract
Background: Cryptococcal meningitis has high mortality. Flucytosine is a key treatment but is expensive and rarely available. The anticancer agent tamoxifen has synergistic anti-cryptococcal activity with amphotericin in vitro. It is off-patent, cheap, and widely available. We performed a trial to determine its therapeutic potential.
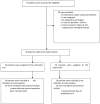
Methods: Open label randomized controlled trial. Participants received standard care - amphotericin combined with fluconazole for the first 2 weeks - or standard care plus tamoxifen 300 mg/day. The primary end point was Early Fungicidal Activity (EFA) - the rate of yeast clearance from cerebrospinal fluid (CSF). Trial registration https://clinicaltrials.gov/ct2/show/NCT03112031.
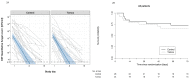
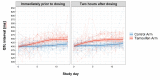
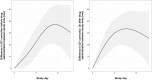
Results: Fifty patients were enrolled (median age 34 years, 35 male). Tamoxifen had no effect on EFA (-0.48log10 colony-forming units/mL/CSF control arm versus -0.49 tamoxifen arm, difference -0.005log10CFU/ml/day, 95% CI: -0.16, 0.15, p=0.95). Tamoxifen caused QTc prolongation.
Conclusions: High-dose tamoxifen does not increase the clearance rate of Cryptococcus from CSF. Novel, affordable therapies are needed.
Funding: The trial was funded through the Wellcome Trust Asia Programme Vietnam Core Grant 106680 and a Wellcome Trust Intermediate Fellowship to JND grant number WT097147MA.
Keywords: HIV; Viet Nam; cryptococcal meningitis; cryptococcus gattii; cryptococcus neoformans; infectious disease; medicine; microbiology; randomised controlled trial.
© 2021, Ngan et al.
Conflict of interest statement
NN, NT, NV, NV, NM, DV, PT, NL, NP, NC, DL, WH, JB, NW, RG, GT, DK, LT, EK, TB, LH, NT, JD No competing interests declared
Figures
References
-
- Ashton PM, Thanh LT, Trieu PH, Van Anh D, Trinh NM, Beardsley J, Kibengo F, Chierakul W, Dance DAB, Rattanavong S, Davong V, Hung LQ, Chau NVV, Tung NLN, Chan AK, Thwaites GE, Lalloo DG, Anscombe C, Nhat LTH, Perfect J, Dougan G, Baker S, Harris S, Day JN. Three phylogenetic groups have driven the recent population expansion of cryptococcus neoformans. Nature Communications. 2019;10:2035. doi: 10.1038/s41467-019-10092-5. - DOI - PMC - PubMed
-
- Beardsley J, Wolbers M, Kibengo FM, Ggayi AB, Kamali A, Cuc NT, Binh TQ, Chau NV, Farrar J, Merson L, Phuong L, Thwaites G, Van Kinh N, Thuy PT, Chierakul W, Siriboon S, Thiansukhon E, Onsanit S, Supphamongkholchaikul W, Chan AK, Heyderman R, Mwinjiwa E, van Oosterhout JJ, Imran D, Basri H, Mayxay M, Dance D, Phimmasone P, Rattanavong S, Lalloo DG, Day JN, CryptoDex Investigators Adjunctive dexamethasone in HIV-Associated cryptococcal meningitis. New England Journal of Medicine. 2016;374:542–554. doi: 10.1056/NEJMoa1509024. - DOI - PMC - PubMed
-
- Bicanic T, Muzoora C, Brouwer AE, Meintjes G, Longley N, Taseera K, Rebe K, Loyse A, Jarvis J, Bekker LG, Wood R, Limmathurotsakul D, Chierakul W, Stepniewska K, White NJ, Jaffar S, Harrison TS. Independent association between rate of clearance of infection and clinical outcome of HIV-associated cryptococcal meningitis: analysis of a combined cohort of 262 patients. Clinical Infectious Diseases. 2009;49:702–709. doi: 10.1086/604716. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

