Using the Alzheimer's Disease Neuroimaging Initiative to improve early detection, diagnosis, and treatment of Alzheimer's disease
- PMID: 34581485
- PMCID: PMC9158456
- DOI: 10.1002/alz.12422
Using the Alzheimer's Disease Neuroimaging Initiative to improve early detection, diagnosis, and treatment of Alzheimer's disease
Abstract
Introduction: The Alzheimer's Disease Neuroimaging Initiative (ADNI) has accumulated 15 years of clinical, neuroimaging, cognitive, biofluid biomarker and genetic data, and biofluid samples available to researchers, resulting in more than 3500 publications. This review covers studies from 2018 to 2020.
Methods: We identified 1442 publications using ADNI data by conventional search methods and selected impactful studies for inclusion.
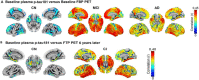
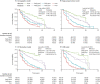
Results: Disease progression studies supported pivotal roles for regional amyloid beta (Aβ) and tau deposition, and identified underlying genetic contributions to Alzheimer's disease (AD). Vascular disease, immune response, inflammation, resilience, and sex modulated disease course. Biologically coherent subgroups were identified at all clinical stages. Practical algorithms and methodological changes improved determination of Aβ status. Plasma Aβ, phosphorylated tau181, and neurofilament light were promising noninvasive biomarkers. Prognostic and diagnostic models were externally validated in ADNI but studies are limited by lack of ethnocultural cohort diversity.
Discussion: ADNI has had a profound impact in improving clinical trials for AD.
Keywords: AV1541 tau positron emission tomography; Alzheimer's disease; amyloid; disease progression; mild cognitive impairment; plasma biomarker; tau.
© 2021 The Authors. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Dr. Veitch was supported for the present work by NIH grant U19‐AG024904 and has no other support or conflicts of interest to declare. Dr. Weiner is the principal investigator of NIH‐funded grants. Over the past 36 months he received funding administered through his institutions (NIH grants: 1RF1AG059009‐01 and 1R01AG058676‐01A1; CA Dept. of Health grant: 19‐10616; NIH Subaward from Dr. Richard Gershon: 1U2CA060426‐01), consulting fees (Cerecin/Accera, Inc., BioClinica, Nestle/Nestec, Roche, Genentech, NIH, The Buck Institute for Research on Aging, FUJIFILM‐Toyama Chemical [Japan], Garfield Weston, Baird Equity Capital, University of Southern California [USC], Cytox, and Japanese Organization for Medical Device Development, Inc. [JOMDD], and T3D Therapeutics), and payment for lecturing (The Buck Institute for Research on Aging). He holds stock options in Anven, Alzheon, and Aleca. He receives other grant support for his work (NIH: 5U19AG024904‐14; 1R01AG053798‐01A1; R01 MH098062; U24 AG057437‐01; 1U2CA060426‐01; 1R01AG058676‐01A1; and 1RF1AG059009‐01, DOD: W81XWH‐15‐2‐0070; 0W81XWH‐12‐2‐0012; W81XWH‐14‐1‐0462; W81XWH‐13‐1‐0259, PCORI: PPRN‐1501‐26817, California Dept. of Public Health: 16‐10054, U. Michigan: 18‐PAF01312, Siemens: 444951‐54249, Biogen: 174552, Hillblom Foundation: 2015‐A‐011‐NET, Alzheimer's Association: BHR‐16‐459161; The State of California: 18‐109929). He also receives support from Johnson & Johnson, Kevin and Connie Shanahan, GE, VUmc, Australian Catholic University (HBI‐BHR), The Stroke Foundation, and the Veterans Administration. He has served on advisory boards for Eli Lilly, Cerecin/Accera, Roche, Alzheon, Inc., Merck Sharp & Dohme Corp., Nestle/Nestec, PCORI/PPRN, Dolby Family Ventures, National Institute on Aging (NIA), Brain Health Registry, and ADNI. He has no other support or conflicts of interest to declare. Dr. Aisen has received support over the last 36 months from payments to his institution from NIA, Alzheimer's Association, Janssen, Lilly, and Eisai. He has served on the advisory boards of Biogen, Merck, Roche, Abbvie, Rainbow Medical, ImmunoBrain Checkpoint, Shionogi, and has no other support or conflicts of interest to declare. Dr. Beckett was supported in the current work by NIA/NIH U01AG024904 to her institution and received support over the last 36 months from payments to her institution from U01AG024904 (Dr. Weiner, UCSF/NCIRE), R01AG062517 (Dr. Dugger), B639943 (Dr. Coleman), and the National Institute of Justice 2014‐R2‐CX‐0012 (Dr. Wintemute). She has served on the external advisory board for Alzheimer's Disease Centers at UCSD, Washington University, University of Pittsburgh and Data and Safety Monitoring Boards for NIH‐funded clinical trial (UCSF), all paid directly to her. Her travel to the AAIC was supported by U01AG024904. She has no other support or conflicts of interest to declare. Dr. DeCarli was supported in the current work by NIH and has received support over the last 36 months from payments to his institution from the NIH. He served on an advisory board at Novartis on a safety study of heart failure treatment and has no other support or conflicts of interest to declare. Dr. Green was supported in the current work by NIH funding. Over the last 36 months, he has received support from multiple NIH grants to his institution, consulting fees (AIA, Genomic Life, Grail, Humanity, Kneed Media, Plumcare, UnitedHealth, Verily, VibrentHealth, and Genome Medical), and lectures in non‐Alzheimer's fields. He has no other support or conflicts of interest to declare. Dr. Harvey has no support or conflicts of interest to declare. Dr. Jack was supported in the current work by NIH funding. Over the last 36 months, he has received support from NIH grants to his institution and has served on the advisory boards of iDMC and Roche with no payments made. He has no other support or conflicts of interest to declare. Dr. Jagust has received support over the last 36 months from grants to his institution (NIH grants R01 AG034570 [Dr. Jagust], R01AG062542 [Dr. Jagust], U24 AG067418 [Dr. Jagust], P01AG019724 [Dr. Bruce Miller], R01 AG031164 [Dr. Matthew Walker], RF1 AG054019 [Dr. Matthew Walker], U01 AG024904 [Dr. Weiner], RF1 AG054106‐01A1 [Dr. Matthew Walker], R44AG046025‐03 [Dr. Daojing Wang], R01 AG061303 [Dr. Lexin Li], MH112775 [Dr. Ming Hsu], 1R01AG062689‐01 [Dr. Landau], AG062624 [Dr. José Luchsinger], and R01AG069090), direct consulting fees (Biogen, Bioclinica, Genentech/Roche, CuraSen, Grifols), and has served on the advisory board of the Alzheimer's Prevention Initiative. He has no other support or conflicts of interest to declare. Dr. Landau has received support over the last 36 months from grants through her institution (R01 AG062689 [Dr. Landau], U19 AG024904 [Dr. Weiner], R01 AG061303 [Dr. Li], R01AG062542 [Dr. Jagust], U24 AG067418 [Dr. Jagust]), an honorarium for speaking (4th annual Hillblom Symposium, University of CA, San Francisco), travel expenses and conference registration (AAIC 2017‐2019 as member of the Scientific Program Committee during this period), and has served on the advisory board of KeifeRx. She has no other support or conflicts of interest to declare. Dr. Morris over the past 36 months received honoraria (Grand Rounds lecture advisory board member) and support to attend national and international meetings, external advisory meetings, and board member meetings. He has no other support or conflicts of interest to declare. Dr. Okonkwo over the past 36 months received grants through his institution from the NIH and served in the International Neuropsychological Society. He has no other support or conflicts of interest to declare. Dr. Perrin over the past 36 months received grants through his institution (U19 AG024904, R01 NS092865, RF1 AG053550, R01 AG054513, R01 AG054567, R01 AG052550, P01 AG00399,1 U19 AG032438, P30 AG066444, U19 AG032438, R01 AG068319, R01AG053267, R01 AG070883, R01 NS103276). He has no other support or conflicts of interest to declare. Dr. Petersen over the past 36 months received grants through his institution (P30 AG062677, U01 AG006786); licenses or royalties from Oxford University Press and UpToDate; and consulting fees from Roche, Merck, Biogen, Genentech, and Eisai. He served on the advisory board of Genentech and has no other support or conflicts of interest to declare. Dr. Rivera‐Mindt over the past 36 months received grants through her institution (NIH/NIA R13 AG071313‐01 [Drs. M. Rivera Mindt, R. Turner‐II, M. Carrillo], Genentech Health Equity Innovations 2020 Fund G‐89294 [Drs. M. Rivera Mindt, R. Nosheny & C. Hill], NIH/NIA R01AG065110 ‐ 01A1 [Dr. Rivera Mindt], NIH/NIA 5U19AG024904‐14 [Dr. Weiner], R01AG066471‐01A1 [Drs. A. Federman & J.P. Wisnivesky], AARGD‐16‐446038 [Dr. Rivera Mindt; PI of subcontract to Mt. Sinai: J. Robinson‐Papp], and NIH/NIMH U24MH100931‐03 [Dr. S. Morgello]), speaking fees for talks at various universities across the country (e.g., Brown, Columbia, University of Arizona), and travel support from NIH grants. She has served on the Society for Black Neuropsychology; is a Present Member, Centers for Disease Control and Prevention (CDC) BOLD Public Health Center of Excellence on Dementia Risk Reduction Expert Panel; and Present Member, CDC/National Alzheimer's Project Act (NAPA) Physical Activity, Tobacco Use, and Alcohol Workgroup; and was paid directly for her role on the 2019 Data Safety & Monitoring Board (DSMB) Member Project Title: Reducing HIV Risk Behavior in Depressed and Non‐Depressed Older Adults with HIV; Grant #: R01AG05308101 (PI: T. Lovejoy). She is a Present Board Member, Alzheimer's Association NYC Chapter Board of Directors, President‐Elect and Past‐President (Elected Position), Hispanic Neuropsychological Society, on the Board of Directors, Harlem Community & Academic Partnership, all unpaid. She has no other support or conflicts of interest to declare. Dr. Saykin was supported in this work by grants from NIH and Department of Defense (NIH grants U01 AG024904, P30 AG010133, R01 AG019771, R01 LM013463, R01 LM011360 and DoD grants W81XWH‐13‐1‐0259 and W81XWH‐12‐2‐0012), and over the past 36 months received support from grants to his institution (as detailed above). He served on the Bayer Oncology Advisory Board and received PET tracer precursor from Avid Radiopharmaceuticals. He has no other support or conflicts of interest to declare. Dr. Shaw has received over the past 36 months grants through his institution (NIH grants U01 AG024904 [ADNI3], UPenn ADRC NIA grant for Biomarker Core; Michael J. Fox Foundation for Parkinson's Research for AD biomarker studies; Roche IIS for AD biomarker studies), fees for the Biogen Teaching program on AD Biomarkers, and travel funds from NIA ADNI3 Biomarker Core. He has served on the Roche Advisory Board, LEADS Advisory board, and Fujirebio Advisory Board. He received in‐kind support from Roche (immunoassay reagents and equipment) for ADNI3. He has no other support or conflicts of interest to declare. Dr. Toga was supported in this work by grants from NIH and has received over the past 36 months grants through his institution from NIH and speaking fees from Biogen. He has no other support or conflicts of interest to declare. Dr. Tosun has received over the past 36 months grants through her institution (NIH U01 AG024904). She has no other support or conflicts of interest to declare. Dr. Trojanowski has received over the past 36 months grants through his institution (AG10124). He has no other support or conflicts of interest to declare.
Figures