The costs of developing treatments for Alzheimer's disease: A retrospective exploration
- PMID: 34581499
- PMCID: PMC8940715
- DOI: 10.1002/alz.12450
The costs of developing treatments for Alzheimer's disease: A retrospective exploration
Abstract
Introduction: With the exception of the recent accelerated approval of aducanumab, in over 26 years of research and development (R&D) investment in Alzheimer's disease (AD), only five novel drugs-all for symptomatic treatment only-have reached FDA approval. Here, we estimate the costs of AD drug development during this period in the private sector.
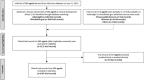
Methods: To estimate private R&D funding, we collected information on AD clinical trials (n = 1099; phases 1-4) conducted between January 1, 1995 and June 21, 2021 from various databases. Costs were derived using previously published methodologies and adjusted for inflation.
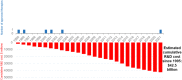
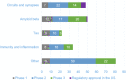
Results: Since 1995, cumulative private expenditures on clinical stage AD R&D were estimated at $42.5 billion, with the greatest costs (57%; $24,065 million) incurred during phase 3; approximately 184,000 participants were registered or are currently enrolled in clinical trials.
Discussion: Measures to reduce expenditures while moving toward disease-modifying therapies that alleviate the rising burden of AD require continued investment from industry, government, and academia.
Keywords: Alzheimer's disease; clinical trials; funding; industry; research and development.
© 2021 Biogen. Alzheimer's & Dementia published by Wiley Periodicals LLC on behalf of Alzheimer's Association.
Conflict of interest statement
Figures


References
-
- Berger JR, Choi D, Kaminski HJ, et al. Importance and hurdles to drug discovery for neurological disease. Ann Neurol. 2013;74:441‐446. - PubMed
-
- Choi DW, Armitage R, Brady LS, et al. Medicines for the mind: policy‐based “pull” incentives for creating breakthrough CNS drugs. Neuron. 2014;84:554‐563. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Medical

