CircRNA-MSR Regulates LPS-Induced C28/I2 Chondrocyte Injury through miR-643/MAP2K6 Signaling Pathway
- PMID: 34581623
- PMCID: PMC8804801
- DOI: 10.1177/19476035211044826
CircRNA-MSR Regulates LPS-Induced C28/I2 Chondrocyte Injury through miR-643/MAP2K6 Signaling Pathway
Abstract
Objective: Osteoarthritis (OA) is a degenerative joint disease characterized by deterioration of articular cartilage functions. Previous studies have confirmed the role of circular RNAs (circRNAs) in OA, but the role of mechanical stress-related circRNA (circRNA-MSR) in OA is unknown.
Design: The human chondrocytes C28/I2 were cultured and treated with lipopolysaccharide (LPS) to establish the OA model. The mRNA and protein levels were measured by qRT-PCR or Western blot. Cell viability was analyzed by MTT assay. Flow cytometry was carried out to detect cell apoptosis. The levels of TNF-α, IL-1β, and IL-6 were determined by enzyme-linked immunosorbent assay (ELISA). Pull-down assay was conducted to measure circRNA-MSR-related miRNA. Dual-luciferase reporter gene detection was performed to detect the target relationships between miR-643 and circRNA-MSR or Mitogen-activated protein kinase kinase 6 (MAP2K6). The RNA-fluorescence in situ hybridization (RNA-FISH) assay was conducted to verify the localization of circRNA-MSR and miR-643.
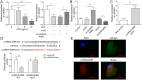
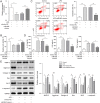
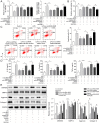
Results: The expressions of circRNA-MSR were upregulated in LPS stimulated C28/I2 cells. Knockdown of circRNA-MSR can inhibit LPS-induced apoptosis, inflammatory response, and extracellular matrix (ECM) degradation, and promote cell C28/I2 cells proliferation. Moreover, circRNA-MSR directly targeted miR-643. RNA-FISH exhibited that circRNA-MSR may act as a competing endogenous RNA (ceRNA) of miR-643. Over-expression of miR-643 could alleviate LPS-induced C28/I2 chondrocyte injury and promote cell proliferation. Besides, miR-643 directly bound to MAP2K6 mRNA. MiR-643 inhibition or MAP2K6 overexpression can reverse the role of circRNA-MSR knockdown on LPS-treated chondrocytes.
Conclusion: circRNA-MSR can upregulate MAP2K6 by targeting miR-643, thereby inhibiting cell proliferation and promoting apoptosis of C28/I2 cells.
Keywords: MAP2K6; circRNA-MSR; miR-643; osteoarthritis.
Conflict of interest statement
Figures



Similar articles
-
circRNA-MSR regulates the expression of FBXO21 to inhibit chondrocyte autophagy by targeting miR-761 in osteoarthritis.Kaohsiung J Med Sci. 2022 Dec;38(12):1168-1177. doi: 10.1002/kjm2.12604. Epub 2022 Oct 24. Kaohsiung J Med Sci. 2022. PMID: 36278814 Free PMC article.
-
[miR-515-5p targeting Toll-like receptor 4 regulates myeloid differentiation primary response gene 88/nuclear factor-kappa B pathway to inhibit apoptosis and inflammatory response of osteoarthritis chondrocytes].Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024 Mar 15;38(3):315-323. doi: 10.7507/1002-1892.202312091. Zhongguo Xiu Fu Chong Jian Wai Ke Za Zhi. 2024. PMID: 38500425 Free PMC article. Chinese.
-
CircTBX5 knockdown modulates the miR-558/MyD88 axis to alleviate IL-1β-induced inflammation, apoptosis and extracellular matrix degradation in chondrocytes via inactivating the NF-κB signaling.J Orthop Surg Res. 2023 Jul 1;18(1):477. doi: 10.1186/s13018-023-03949-5. J Orthop Surg Res. 2023. PMID: 37393232 Free PMC article.
-
Knockdown of lncRNA MFI2-AS1 inhibits lipopolysaccharide-induced osteoarthritis progression by miR-130a-3p/TCF4.Life Sci. 2020 Jan 1;240:117019. doi: 10.1016/j.lfs.2019.117019. Epub 2019 Oct 31. Life Sci. 2020. PMID: 31678554 Review.
-
CircRNA-mediated ceRNA mechanism in Osteoarthritis: Special emphasis on circRNAs in exosomes and the crosstalk of circRNAs and RNA methylation.Biochem Pharmacol. 2023 Jun;212:115580. doi: 10.1016/j.bcp.2023.115580. Epub 2023 May 5. Biochem Pharmacol. 2023. PMID: 37148980 Review.
Cited by
-
CircRNAs in osteoarthritis: research status and prospect.Front Genet. 2023 May 9;14:1173812. doi: 10.3389/fgene.2023.1173812. eCollection 2023. Front Genet. 2023. PMID: 37229197 Free PMC article. Review.
-
circRNA-MSR regulates the expression of FBXO21 to inhibit chondrocyte autophagy by targeting miR-761 in osteoarthritis.Kaohsiung J Med Sci. 2022 Dec;38(12):1168-1177. doi: 10.1002/kjm2.12604. Epub 2022 Oct 24. Kaohsiung J Med Sci. 2022. PMID: 36278814 Free PMC article.
-
ZBTB16 eases lipopolysaccharide‑elicited inflammation, apoptosis and degradation of extracellular matrix in chondrocytes during osteoarthritis by suppressing GRK2 transcription.Exp Ther Med. 2023 Apr 24;25(6):276. doi: 10.3892/etm.2023.11975. eCollection 2023 Jun. Exp Ther Med. 2023. PMID: 37206562 Free PMC article.
-
Exercise for Osteoarthritis: A Literature Review of Pathology and Mechanism.Front Aging Neurosci. 2022 May 3;14:854026. doi: 10.3389/fnagi.2022.854026. eCollection 2022. Front Aging Neurosci. 2022. PMID: 35592699 Free PMC article. Review.
-
CircRELL1 promotes osteoarthritis progression by regulating miR-200c-3p.Heliyon. 2024 Jul 6;10(14):e34251. doi: 10.1016/j.heliyon.2024.e34251. eCollection 2024 Jul 30. Heliyon. 2024. PMID: 39130448 Free PMC article.
References
-
- Browne JL, Hooshmand S, Elam M, Fe Resin R, Arjmandi B. Relationship between inflammation, oxidative stress, and oxidative damage with severity of knee osteoarthritis (OA). Phytomed Int J Phytother Phytopharmacol. 2012;20:470-80.
-
- Xu Q, Chen B, Wang Y, Wang X, Han D, Ding D, et al.. The effectiveness of manual therapy for relieving pain, stiffness and dysfunction in knee osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2015;23:A387. - PubMed
-
- Thomas CM, Fuller CJ, Whittles CE, Sharif M. Chondrocyte death by apoptosis is associated with cartilage matrix degradation. Osteoarthritis Cartilage. 2007;15:27-34. - PubMed
-
- Liu Q, Zhao J, Tan R, Zhou H, Lin Z, Zheng M, et al.. Parthenolide inhibits pro-inflammatory cytokine production and exhibits protective effects on progression of collagen-induced arthritis in a rat model. Scand J Rheumatol. 2015;44:182-91. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

