Age-dependent changes in protein incorporation into collagen-rich tissues of mice by in vivo pulsed SILAC labelling
- PMID: 34581667
- PMCID: PMC8478409
- DOI: 10.7554/eLife.66635
Age-dependent changes in protein incorporation into collagen-rich tissues of mice by in vivo pulsed SILAC labelling
Abstract
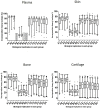
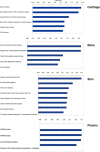
Collagen-rich tissues have poor reparative capacity that predisposes to common age-related disorders such as osteoporosis and osteoarthritis. We used in vivo pulsed SILAC labelling to quantify new protein incorporation into cartilage, bone, and skin of mice across the healthy life course. We report dynamic turnover of the matrisome, the proteins of the extracellular matrix, in bone and cartilage during skeletal maturation, which was markedly reduced after skeletal maturity. Comparing young adult with older adult mice, new protein incorporation was reduced in all tissues. STRING clustering revealed changes in epigenetic modulators across all tissues, a decline in chondroprotective growth factors such as FGF2 and TGFβ in cartilage, and clusters indicating mitochondrial dysregulation and reduced collagen synthesis in bone. Several pathways were implicated in age-related disease. Fewer changes were observed for skin. This methodology provides dynamic protein data at a tissue level, uncovering age-related molecular changes that may predispose to disease.
Keywords: Age; SILAC; cell biology; collagen-rich tissues; matrisome; mouse; protein dynamics; proteomics.
© 2021, Ariosa-Morejon et al.
Conflict of interest statement
YA, AS, RF, SD, RT, AW, TV none, PC None
Figures










References
-
- Chia SL, Sawaji Y, Burleigh A, McLean C, Inglis J, Saklatvala J, Vincent T. Fibroblast growth factor 2 is an intrinsic chondroprotective agent that suppresses ADAMTS-5 and delays cartilage degradation in murine osteoarthritis. Arthritis and Rheumatism. 2009;60:2019–2027. doi: 10.1002/art.24654. - DOI - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources

