LPHN2 inhibits vascular permeability by differential control of endothelial cell adhesion
- PMID: 34581723
- PMCID: PMC8480966
- DOI: 10.1083/jcb.202006033
LPHN2 inhibits vascular permeability by differential control of endothelial cell adhesion
Abstract
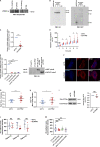
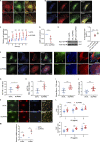
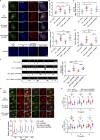
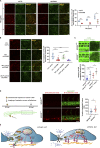
Dynamic modulation of endothelial cell-to-cell and cell-to-extracellular matrix (ECM) adhesion is essential for blood vessel patterning and functioning. Yet the molecular mechanisms involved in this process have not been completely deciphered. We identify the adhesion G protein-coupled receptor (ADGR) Latrophilin 2 (LPHN2) as a novel determinant of endothelial cell (EC) adhesion and barrier function. In cultured ECs, endogenous LPHN2 localizes at ECM contacts, signals through cAMP/Rap1, and inhibits focal adhesion (FA) formation and nuclear localization of YAP/TAZ transcriptional regulators, while promoting tight junction (TJ) assembly. ECs also express an endogenous LPHN2 ligand, fibronectin leucine-rich transmembrane 2 (FLRT2), that prevents ECM-elicited EC behaviors in an LPHN2-dependent manner. Vascular ECs of lphn2a knock-out zebrafish embryos become abnormally stretched, display a hyperactive YAP/TAZ pathway, and lack proper intercellular TJs. Consistently, blood vessels are hyperpermeable, and intravascularly injected cancer cells extravasate more easily in lphn2a null animals. Thus, LPHN2 ligands, such as FLRT2, may be therapeutically exploited to interfere with cancer metastatic dissemination.
© 2021 Camillo et al.
Figures




Similar articles
-
Cell adhesion controlled by adhesion G protein-coupled receptor GPR124/ADGRA2 is mediated by a protein complex comprising intersectins and Elmo-Dock.J Biol Chem. 2017 Jul 21;292(29):12178-12191. doi: 10.1074/jbc.M117.780304. Epub 2017 Jun 9. J Biol Chem. 2017. PMID: 28600358 Free PMC article.
-
Flow-dependent YAP/TAZ activities regulate endothelial phenotypes and atherosclerosis.Proc Natl Acad Sci U S A. 2016 Oct 11;113(41):11525-11530. doi: 10.1073/pnas.1613121113. Epub 2016 Sep 26. Proc Natl Acad Sci U S A. 2016. PMID: 27671657 Free PMC article.
-
TNF-α-Induced YAP/TAZ Activity Mediates Leukocyte-Endothelial Adhesion by Regulating VCAM1 Expression in Endothelial Cells.Int J Mol Sci. 2018 Nov 1;19(11):3428. doi: 10.3390/ijms19113428. Int J Mol Sci. 2018. PMID: 30388809 Free PMC article.
-
Flow-Dependent Endothelial YAP Regulation Contributes to Vessel Maintenance.Dev Cell. 2017 Mar 27;40(6):523-536.e6. doi: 10.1016/j.devcel.2017.02.019. Dev Cell. 2017. PMID: 28350986
-
Mechanisms of increased endothelial permeability.Can J Physiol Pharmacol. 1996 Jul;74(7):787-800. doi: 10.1139/y96-081. Can J Physiol Pharmacol. 1996. PMID: 8946065 Review.
Cited by
-
Cartography of teneurin and latrophilin expression reveals spatiotemporal axis heterogeneity in the mouse hippocampus during development.PLoS Biol. 2024 May 7;22(5):e3002599. doi: 10.1371/journal.pbio.3002599. eCollection 2024 May. PLoS Biol. 2024. PMID: 38713721 Free PMC article.
-
Fibronectin leucine-rich transmembrane protein 2 drives monocyte differentiation into macrophages via the UNC5B-Akt/mTOR axis.Front Immunol. 2023 Apr 6;14:1162004. doi: 10.3389/fimmu.2023.1162004. eCollection 2023. Front Immunol. 2023. PMID: 37090697 Free PMC article.
-
Identification of a subpopulation of highly adherent endothelial cells for seeding synthetic vascular grafts.J Thorac Cardiovasc Surg. 2025 Jul;170(1):e27-e43. doi: 10.1016/j.jtcvs.2024.06.028. Epub 2024 Jul 5. J Thorac Cardiovasc Surg. 2025. PMID: 38972570
-
Amphiphilic and fatigue-resistant organohydrogels for small-diameter vascular grafts.Sci Adv. 2022 Jul 29;8(30):eabn5360. doi: 10.1126/sciadv.abn5360. Epub 2022 Jul 29. Sci Adv. 2022. PMID: 35905180 Free PMC article.
-
How to Generate a Vascular-Labelled Transgenic Zebrafish Model to Study Tumor Angiogenesis and Extravasation.Methods Mol Biol. 2023;2572:191-202. doi: 10.1007/978-1-0716-2703-7_15. Methods Mol Biol. 2023. PMID: 36161418
References
-
- Avanzato, D., Genova T., Fiorio Pla A., Bernardini M., Bianco S., Bussolati B., Mancardi D., Giraudo E., Maione F., Cassoni P., et al. . 2016. Activation of P2X7 and P2Y11 purinergic receptors inhibits migration and normalizes tumor-derived endothelial cells via cAMP signaling. Sci. Rep. 6:32602. 10.1038/srep32602 - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Associated data
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases

