The promise and perils of immunotherapy
- PMID: 34581774
- PMCID: PMC8945582
- DOI: 10.1182/bloodadvances.2021004453C
The promise and perils of immunotherapy
Abstract
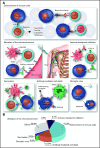
Advances in understanding the ways in which the immune system fails to control tumor growth or prevent autoimmunity have led to the development of powerful therapeutic strategies to treat these diseases. In contrast to conventional therapies that have a broadly suppressive effect, immunotherapies are more akin to targeted therapies because they are mechanistically driven and are typically developed with the goal of "drugging" a specific underlying pathway or phenotype. This means that their effects and toxicities are, at least in theory, more straightforward to anticipate. The development of functionalized antibodies, genetically engineered T cells, and immune checkpoint inhibitors continues to accelerate, illuminating new biology and bringing new treatment to patients. In the following sections, we provide an overview of immunotherapeutic concepts, highlight recent advances in the field of immunotherapies, and discuss controversies and future directions, particularly as these pertain to hematologic oncology or blood-related diseases. We conclude by illustrating how original research published in this journal fits into and contributes to the overall framework of advances in immunotherapy.
© 2021 by The American Society of Hematology.
Figures
References
-
- Kasamon YL, Chen H, de Claro RA, et al. . FDA approval summary: mogamulizumab-kpkc for mycosis fungoides and Sézary syndrome. Clin Cancer Res. 2019;25(24):7275-7280. - PubMed
-
- Salles G, Duell J, González Barca E, et al. . Tafasitamab plus lenalidomide in relapsed or refractory diffuse large B-cell lymphoma (L-MIND): a multicentre, prospective, single-arm, phase 2 study. Lancet Oncol. 2020;21(7):978-988. - PubMed
-
- Coiffier B, Haioun C, Ketterer N, et al. . Rituximab (anti-CD20 monoclonal antibody) for the treatment of patients with relapsing or refractory aggressive lymphoma: a multicenter phase II study. Blood. 1998;92(6):1927-1932. - PubMed
-
- Coiffier B, Lepage E, Briere J, et al. . CHOP chemotherapy plus rituximab compared with CHOP alone in elderly patients with diffuse large-B-cell lymphoma. N Engl J Med. 2002;346(4):235-242. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

