A novel patient-reported outcome instrument assessing the symptoms of paroxysmal nocturnal hemoglobinuria, the PNH-SQ
- PMID: 34581910
- PMCID: PMC8479131
- DOI: 10.1186/s41687-021-00376-0
A novel patient-reported outcome instrument assessing the symptoms of paroxysmal nocturnal hemoglobinuria, the PNH-SQ
Abstract
Background: Patient-reported outcome measures (PROs) used to measure symptoms of patients with paroxysmal nocturnal hemoglobinuria (PNH) in trials do not measure PNH symptoms comprehensively and do not assess daily fluctuations in symptoms. Following a literature review and consultation with a PNH expert, we drafted the PNH Symptom Questionnaire (PNH-SQ) and a patient-centric conceptual model of PNH symptoms and impacts. We then interviewed 15 patients with PNH to assess comprehensiveness of symptom capture from the patient perspective and to cognitively debrief the PNH-SQ. Patient interview data were also used to finalize the PNH conceptual model.
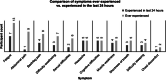
Results: Participants mentioned 27 signs or symptoms of PNH spontaneously or after being probed; 93% reported experiencing ≥ 1 PNH symptom. Concept saturation was reached for all PNH symptoms. Further, interviews confirmed the instrument captured the most common PNH symptoms, including fatigue (87%), abdominal pain (60%), and difficulty swallowing (47%), with fatigue ranked as the most bothersome symptom. The interviews demonstrated that participants understood the items of the PNH-SQ (90-100%); considered the symptoms relevant (> 50- > 90%); the recall period appropriate (> 80-100%); and the response options suitable (> 80-100%). Participants also suggested changes regarding item redundancy and relevance; this feedback was used to finalize the instrument.
Conclusions: The finalized PNH-SQ assesses the presence and severity of 10 symptoms-abdominal pain, chest discomfort, difficulty sleeping, difficulty swallowing, difficulty thinking clearly, fatigue, headache, muscle weakness, pain in the legs or back, and shortness of breath-over 24 h. The PNH-SQ is a content-valid questionnaire suitable for assessing daily symptom presence and severity in PNH clinical trials.
Keywords: PNH; PRO; QoL; Symptom.
© 2021. The Author(s).
Conflict of interest statement
R.P.D. was an employee of Clinical Outcomes Solutions; J.J.J. is an employee of Regeneron Pharmaceuticals, Inc.; S.K. and T.S. are employees of Clinical Outcomes Solutions; J.S. has the following disclosures: Regeneron Pharmaceuticals, Inc.: Consultancy; Incyte: Consultancy, Honoraria, Research Funding, Speakers Bureau; CTI Pharma: Research Funding; Onconova: Research Funding; Alexion: Consultancy, Honoraria, Research Funding, Speakers Bureau; Celgene: Consultancy, Honoraria, Research Funding, Speakers Bureau; Apellis: Membership on an entity's Board of Directors or advisory committees; Otsuka: Consultancy, Honoraria; Novartis: Consultancy, Honoraria; Astex Pharma: Research Funding; Sanofi: Consultancy, Honoraria, Speakers Bureau.
Figures
References
-
- Mitchell R, et al. Path to diagnosis of paroxysmal nocturnal hemoglobinuria: the results of an exploratory study conducted by the aplastic anemia and MDS international foundation and the national organization for rare disorders utilizing an internet-based survey. SM Clin Med Oncol. 2017;1(1):1001.
-
- Hill A, et al. The incidence and prevalence of paroxysmal nocturnal hemoglobinuria (PNH) and survival of patients in Yorkshire. Washington: American Society of Hematology; 2006.
-
- Jalbert JJ, et al. Epidemiology of PNH and real-world treatment patterns following an incident PNH diagnosis in the US. Washington: American Society of Hematology; 2019.
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous