Integration of in vitro allergy test results and ratio analysis for the diagnosis and treatment of allergic patients (INTEGRA)
- PMID: 34582103
- PMCID: PMC9082998
- DOI: 10.1002/clt2.12052
Integration of in vitro allergy test results and ratio analysis for the diagnosis and treatment of allergic patients (INTEGRA)
Abstract
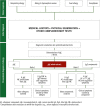
The introduction of molecular diagnosis into routine clinical practice has substantially improved the diagnosis and management of allergic patients by allowing clinicians to precisely identify the allergenic molecule responsible for immunoglobulin E (IgE)-mediated allergies. However, it can be challenging to accurately interpret the results of molecular assays, partly due to the limited evidence base. In this context, a panel of experts with extensive experience in interpreting in vitro measures of total and serum specific IgE reviewed the available scientific evidence. After this review, the panel selected a series of representative case studies to demonstrate how determination of specific and total IgE values and the relationship between them (ratio analysis) can add value to the diagnostic process by more precisely defining the patient's sensitization profile. Finally, the experts developed a series of recommendations on the clinical application of ratio analysis to optimize and complement the classical approach to allergy diagnosis.
Keywords: IgE; IgE ratio; allergy; molecular diagnosis; recommendations.
© 2021 The Authors. Clinical and Translational Allergy published by John Wiley & Sons Ltd on behalf of European Academy of Allergy and Clinical Immunology.
Conflict of interest statement
The authors have received consulting fees and financial support from Thermo Fisher Scientific to attend workshops related to the preparation of this article.
Figures
References
-
- Manifiesto EAACI Advocacy . Tackling the Allergy Crisis in Europe – Concerted Policy Action Needed. European Academy of Allergy and Clinical Immunology; 2015.
-
- Matricardi PM, Kleine‐Tebbe J, Hoffmann HJ, et al. EAACI molecular allergology user's guide. Pediatr Allergy Immunol. 2016;27(Suppl 23):1‐250. - PubMed

