DNA vaccines for SARS-CoV-2: toward third-generation vaccination era
- PMID: 34582298
- PMCID: PMC8567274
- DOI: 10.1080/14760584.2021.1987223
DNA vaccines for SARS-CoV-2: toward third-generation vaccination era
Abstract
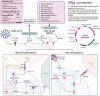
Introduction: Coronavirus outbreak 2019 (COVID-19) has affected all the corners of the globe and created chaos to human life. In order to put some control on the pandemic, vaccines are urgently required that are safe, cost effective, easy to produce, and most importantly induce appropriate immune responses and protection against viral infection. DNA vaccines possess all these features and are promising candidates for providing protection against SARS-CoV-2.Area covered: Current understanding and advances in DNA vaccines toward COVID-19, especially those under various stages of clinical trials.Expert opinion: Through DNA vaccines, host cells are momentarily transformed into factories that produce proteins of the SARS-CoV-2. The host immune system detects these proteins to develop antibodies that neutralize and prevent the infection. This vaccine platform has additional benefits compared to traditional vaccination strategies like strong cellular immune response, higher safety margin, a simple production process as per cGMP norms, lack of any infectious agent, and a robust platform for large-scale production.
Keywords: COVID-19; DNA vaccine; SARS-CoV-2; genetic vaccine; immunization; nucleotide vaccines; pandemic; vaccine.
Figures

References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Other Literature Sources
Medical
Miscellaneous
