ANGPTL8 in metabolic homeostasis: more friend than foe?
- PMID: 34582711
- PMCID: PMC8478524
- DOI: 10.1098/rsob.210106
ANGPTL8 in metabolic homeostasis: more friend than foe?
Abstract
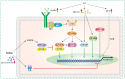
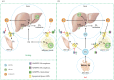
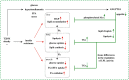
ANGPTL8 is an important cytokine, which is significantly increased in type 2 diabetes mellitus (T2DM), obesity and metabolic syndrome. Many studies have shown that ANGPTL8 can be used as a bio-marker of these metabolic disorders related diseases, and the baseline ANGPTL8 level has also been found to be positively correlated with retinopathy and all-cause mortality in patients with T2DM. This may be related to the inhibition of lipoprotein lipase activity and the reduction of circulating triglyceride (TG) clearance by ANGPTL8. Consistently, inhibition of ANGPTL8 seems to prevent or improve atherosclerosis. However, it is puzzling that ANGPTL8 seems to have a directing function for TG uptake in peripheral tissues; that is, ANGPTL8 specifically enhances the reserve and buffering function of white adipose tissue, which may alleviate the ectopic lipid accumulation to a certain extent. Furthermore, ANGPTL8 can improve insulin sensitivity and inhibit hepatic glucose production. These contradictory results lead to different opinions on the role of ANGPTL8 in metabolic disorders. In this paper, the correlation between ANGPTL8 and metabolic diseases, the regulation of ANGPTL8 and the physiological role of ANGPTL8 in the process of glucose and lipid metabolism were summarized, and the physiological/pathological significance of ANGPTL8 in the process of metabolic disorder was discussed.
Keywords: ANGPTL8; T2DM; betatrophin; glucose metabolism; lipid metabolism.
Conflict of interest statement
All authors have nothing to declare.
Figures
Similar articles
-
ANGPTL8 (betatrophin) role in diabetes and metabolic diseases.Diabetes Metab Res Rev. 2017 Nov;33(8). doi: 10.1002/dmrr.2919. Epub 2017 Aug 30. Diabetes Metab Res Rev. 2017. PMID: 28722798 Review.
-
Analysis of the Expression and Prognostic Potential of a Novel Metabolic Regulator ANGPTL8/Betatrophin in Human Cancers.Pathol Oncol Res. 2021 Sep 27;27:1609914. doi: 10.3389/pore.2021.1609914. eCollection 2021. Pathol Oncol Res. 2021. PMID: 34646087 Free PMC article.
-
Regulation of angiopoietin-like protein 8 expression under different nutritional and metabolic status.Endocr J. 2019 Dec 25;66(12):1039-1046. doi: 10.1507/endocrj.EJ19-0263. Epub 2019 Oct 19. Endocr J. 2019. PMID: 31631098 Review.
-
Mice lacking ANGPTL8 (Betatrophin) manifest disrupted triglyceride metabolism without impaired glucose homeostasis.Proc Natl Acad Sci U S A. 2013 Oct 1;110(40):16109-14. doi: 10.1073/pnas.1315292110. Epub 2013 Sep 16. Proc Natl Acad Sci U S A. 2013. PMID: 24043787 Free PMC article.
-
Zinc finger protein ZNF638 regulates triglyceride metabolism via ANGPTL8 in an estrogen dependent manner.Metabolism. 2024 Mar;152:155784. doi: 10.1016/j.metabol.2024.155784. Epub 2024 Jan 9. Metabolism. 2024. PMID: 38211696
Cited by
-
Sugar and Dyslipidemia: A Double-Hit, Perfect Storm.J Clin Med. 2023 Aug 31;12(17):5660. doi: 10.3390/jcm12175660. J Clin Med. 2023. PMID: 37685728 Free PMC article. Review.
-
The chylomicron saga: time to focus on postprandial metabolism.Front Endocrinol (Lausanne). 2024 Jan 18;14:1322869. doi: 10.3389/fendo.2023.1322869. eCollection 2023. Front Endocrinol (Lausanne). 2024. PMID: 38303975 Free PMC article. Review.
-
Alterations in serum concentrations of visfatin and betatrophin in dogs with hypothyroidism.J Vet Intern Med. 2023 Nov-Dec;37(6):2064-2072. doi: 10.1111/jvim.16904. Epub 2023 Oct 20. J Vet Intern Med. 2023. PMID: 37864301 Free PMC article.
-
TDAG51 Attenuates Impaired Lipid Metabolism and Insulin Resistance in Gestational Diabetes Mellitus Through SREBP-1/ANGPTL8 Pathway.Balkan Med J. 2023 May 8;40(3):175-181. doi: 10.4274/balkanmedj.galenos.2023.2022-8-61. Epub 2023 Mar 24. Balkan Med J. 2023. PMID: 36960944 Free PMC article.
-
ANGPTL8 deficiency attenuates lipopolysaccharide-induced liver injury by improving lipid metabolic dysregulation.J Lipid Res. 2024 Aug;65(8):100595. doi: 10.1016/j.jlr.2024.100595. Epub 2024 Jul 15. J Lipid Res. 2024. PMID: 39019343 Free PMC article.
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

