Update on SARS-CoV-2 seroprevalence: regional and worldwide
- PMID: 34582980
- PMCID: PMC8548624
- DOI: 10.1016/j.cmi.2021.09.019
Update on SARS-CoV-2 seroprevalence: regional and worldwide
Abstract
Background: With limited vaccine supplies, an informed position on the status of SARS-CoV-2 infection in people can assist the prioritization of vaccine deployment.
Objectives: We performed a systematic review and meta-analysis to estimate the global and regional SARS-CoV-2 seroprevalences around the world.
Data sources: We systematically searched peer-reviewed databases (PubMed, Embase and Scopus), and preprint servers (medRxiv, bioRxiv and SSRN) for articles published between 1 January 2020 and 30 March 2021.
Study eligibility criteria: Population-based studies reporting the SARS-CoV-2 seroprevalence in the general population were included.
Participants: People of different age groups, occupations, educational levels, ethnic backgrounds and socio-economic status from the general population.
Interventions: There were no interventions.
Methods: We used the random-effects meta-analyses and empirical Bayesian method to estimate the pooled seroprevalence and conducted subgroup and meta-regression analyses to explore potential sources of heterogeneity as well as the relationship between seroprevalence and socio-demographics.
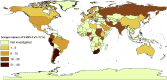
Results: We identified 241 eligible studies involving 6.3 million individuals from 60 countries. The global pooled seroprevalence was 9.47% (95% CI 8.99-9.95%), although the heterogeneity among studies was significant (I2 = 99.9%). We estimated that ∼738 million people had been infected with SARS-CoV-2 (as of December 2020). Highest and lowest seroprevalences were recorded in Central and Southern Asia (22.91%, 19.11-26.72%) and Eastern and South-eastern Asia (1.62%, 1.31-1.95%), respectively. Seroprevalence estimates were higher in males, persons aged 20-50 years, in minority ethnic groups living in countries or regions with low income and human development indices.
Conclusions: The present study indicates that the majority of the world's human population was still highly susceptible to SARS-CoV-2 infection in mid-2021, emphasizing the need for vaccine deployment to vulnerable groups of people, particularly in developing countries, and for the implementation of enhanced preventive measures until 'herd immunity' to SARS-CoV-2 has developed.
Keywords: General population; Meta-analysis; SARS-CoV-2; Seroprevalence; Serum antibodies; Subgroup analyses.
Copyright © 2021 European Society of Clinical Microbiology and Infectious Diseases. Published by Elsevier Ltd. All rights reserved.
Figures



References
-
- Hartley D.M., Perencevich E.N. Public health interventions for COVID-19: emerging evidence and implications for an evolving public health crisis. JAMA. 2020;323:1908–1909. - PubMed
-
- World Health Organization (WHO) COVID-19 Weekly Epidemiological Update-24 August 202. https://www.who.int/publications/m/item/weekly-epidemiological-update-on... Available from:
-
- Institute for Health Metrics and Evaluation (IHME) IHME, University of Washington; Seattle, USA: 2021. COVID-19 results briefing:[global]https://www.healthdata.org/covid/updates Available from:
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

