Photosynthesis from stolen chloroplasts can support sea slug reproductive fitness
- PMID: 34583582
- PMCID: PMC8479339
- DOI: 10.1098/rspb.2021.1779
Photosynthesis from stolen chloroplasts can support sea slug reproductive fitness
Abstract
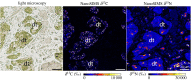
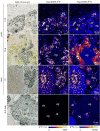
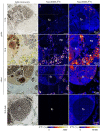
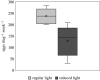
Some sea slugs are able to steal functional chloroplasts (kleptoplasts) from their algal food sources, but the role and relevance of photosynthesis to the animal host remain controversial. While some researchers claim that kleptoplasts are slowly digestible 'snacks', others advocate that they enhance the overall fitness of sea slugs much more profoundly. Our analysis shows light-dependent incorporation of 13C and 15N in the albumen gland and gonadal follicles of the sea slug Elysia timida, representing translocation of photosynthates to kleptoplast-free reproductive organs. Long-chain polyunsaturated fatty acids with reported roles in reproduction were produced in the sea slug cells using labelled precursors translocated from the kleptoplasts. Finally, we report reduced fecundity of E. timida by limiting kleptoplast photosynthesis. The present study indicates that photosynthesis enhances the reproductive fitness of kleptoplast-bearing sea slugs, confirming the biological relevance of this remarkable association between a metazoan and an algal-derived organelle.
Keywords: Sacoglossa; fatty acid; kleptoplast; reproduction.
Figures

References
Publication types
MeSH terms
Associated data
LinkOut - more resources
Full Text Sources
Research Materials
