HAE patient self-sampling for biomarker establishment
- PMID: 34583739
- PMCID: PMC8478266
- DOI: 10.1186/s13023-021-02021-x
HAE patient self-sampling for biomarker establishment
Abstract
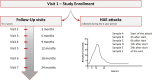
Background: Hereditary Angioedema (HAE) is a genetic disorder that leads to frequent angioedema attacks in various parts of the body. In most cases it is caused by pathogenic variants in the SERPING1 gene, coding for C1-Inhibitor (C1-INH). The pathogenic variants in the gene result in reduced C1-INH levels and/or activity, which causes aberrant bradykinin production and enhanced vascular permeability. The standard-of-care diagnostic test is performed biochemically via measuring C1-INH level and activity as well as the C4 level. This, however, does not allow for the diagnosis of HAE types with normal C1-INH. There is an urgent need to identify and characterize HAE biomarkers for facilitating diagnostics and personalizing the treatment. The Hereditary Angioedema Kininogen Assay (HAEKA) study aims to measure the dynamics of cleaved High Molecular Weight Kininogen (HKa) and other metabolite levels during the angioedema and non-angioedema state of the disease. The metabolites will be analyzed and verified by liquid chromatography ion mobility high resolution mass spectrometry (LC/IM-QToF MS) of dried blood spot (DBS) cards upon the study completion. The study design is truly innovative: 100 enrolled participants provide blood samples via DBS: (1) every 3 months within 2 years during regular study site visits and (2) by at-home self-sampling during HAE attacks via finger pricking. We are presenting a project design that permits clinical study activities during pandemic contact restrictions and opens the door for other clinical studies during COVID-19.
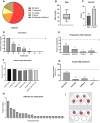
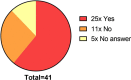
Results: As of October 2020, there are 41 patients from 5 sites in Germany enrolled. 90 blood samples were collected during the regular visits, and 19 of the participants also performed self-sampling during the HAE attacks from which a total of 286 attack blood samples were collected. Participating patients rate the study procedures as easy to implement in their daily lives. The concept of home self-sampling is effective, reproducible, and convenient especially in times of contact restrictions due to the COVID-19 pandemic.
Conclusions: It is the hope that the HAEKA study will complete in 2023, reveal biomarker(s) for monitoring HAE disease activity, and may help to avoid HAE attacks via applying medication prior to the symptom onset.
Keywords: Biomarker; Cleaved high molecular weight Kininogen; Hereditary angioedema; Observational clinical study; Self-sampling.
© 2021. The Author(s).
Conflict of interest statement
Toni M. Förster, Arndt Rolfs, and Volha Skrahina have been during the concept phase and initiation of the study CENTOGENE GmbH employees. Arndt Rolfs has been the founder of CENTOGENE GmbH and holds stock/stock options in the company. Selen Zülbahar and Susanne Zielke are CENTOGENE GmbH employees. Marcus Maurer reports personal fees from Alnylam, grants and personal fees from BioCryst, grants from CENTOGENE, grants and personal fees from CSL Behring, grants from Dyax, grants and personal fees from Kalvista, grants from Pharming, personal fees from Pharvaris, grants and personal fees from Shire/Takeda, outside the submitted work. Markus Magerl is a PI of a center of the HAEKA study. He further reports personal fees and non-financial support from Shire Takeda, personal fees and non-financial support from CSL Behring, personal fees from Pharming, personal fees and non-financial support from Biocryst, personal fees from Kalvista, personal fees from Octapharma, outside the submitted work. Donatello Crocetta is employee of Takeda. Neil Inhaber is employee of Takeda and holds stock/stock options in Takeda.
Figures
References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

