Paclitaxel Induces Micronucleation and Activates Pro-Inflammatory cGAS-STING Signaling in Triple-Negative Breast Cancer
- PMID: 34583980
- PMCID: PMC8643310
- DOI: 10.1158/1535-7163.MCT-21-0195
Paclitaxel Induces Micronucleation and Activates Pro-Inflammatory cGAS-STING Signaling in Triple-Negative Breast Cancer
Abstract
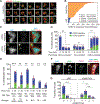
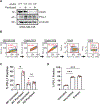
Taxanes remain one of the most effective medical treatments for breast cancer. Clinical trials have coupled taxanes with immune checkpoint inhibitors in patients with triple-negative breast cancer (TNBC) with promising results. However, the mechanism linking taxanes to immune activation is unclear. To determine if paclitaxel could elicit an antitumoral immune response, we sampled tumor tissues from patients with TNBC receiving weekly paclitaxel (80 mg/m2) and found increased stromal tumor-infiltrating lymphocytes and micronucleation over baseline in three of six samples. At clinically relevant concentrations, paclitaxel can induce chromosome missegregation on multipolar spindles during mitosis. Consequently, post-mitotic cells are multinucleated and contain micronuclei, which often activate cyclic GMP-AMP synthase (cGAS) and may induce a type I IFN response reliant on the stimulator of IFN genes (STING) pathway. Other microtubule-targeting agents, eribulin and vinorelbine, recapitulate this cGAS/STING response and increased the expression of immune checkpoint molecule, PD-L1, in TNBC cell lines. To test the possibility that microtubule-targeting agents sensitize tumors that express cGAS to immune checkpoint inhibitors, we identified 10 patients with TNBC treated with PD-L1 or PD-1, seven of whom also received microtubule-targeting agents. Elevated baseline cGAS expression significantly correlated with treatment response in patients receiving microtubule-targeting agents in combination with immune checkpoint inhibitors. Our study identifies a mechanism by which microtubule-targeting agents can potentiate an immune response in TNBC. Further, baseline cGAS expression may predict patient treatment response to therapies combining microtubule-targeting agents and immune checkpoint inhibitors.
©2021 American Association for Cancer Research.
Figures



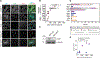
References
-
- Kellokumpu-Lehtinen P, Tuunanen T, Asola R, Elomaa L, Heikkinen M, Kokko R, et al. Weekly paclitaxel--an effective treatment for advanced breast cancer. Anticancer Res. 2013;33:2623–7. - PubMed
-
- Schmid P, Adams S, Rugo HS, Schneeweiss A, Barrios CH, Iwata H, et al. IMpassion130: updated overall survival (OS) from a global, randomized, double-blind, placebo-controlled, Phase III study of atezolizumab (atezo) + nab- paclitaxel (nP) in previously untreated locally advanced or metastatic triple-negative breast cancer (mTNBC). JCO. 2019;37:1003–1003.
-
- Cortes J, Cescon DW, Rugo HS, Nowecki Z, Im S-A, Yusof MM, et al. Pembrolizumab plus chemotherapy versus placebo plus chemotherapy for previously untreated locally recurrent inoperable or metastatic triple-negative breast cancer (KEYNOTE-355): a randomised, placebo-controlled, double-blind, phase 3 clinical trial. The Lancet. Elsevier; 2020;396:1817–28. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

