Enhancing esophageal repair with bioactive bilayer mesh containing FGF
- PMID: 34584186
- PMCID: PMC8478899
- DOI: 10.1038/s41598-021-98840-w
Enhancing esophageal repair with bioactive bilayer mesh containing FGF
Abstract
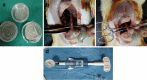
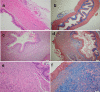
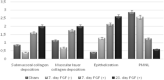
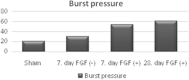
We aimed to prepare a bioactive and biodegradable bilayer mesh formed by fibroblast growth factor (FGF) loaded gelatin film layer, and poly ε-caprolactone (PCL) film layer, and to investigate its treatment efficacy on esophageal anastomosis. It is envisaged that the bioactive mesh in in vivo model would improve tissue healing in rats. The full thickness semicircular defects of 0.5 × 0.5 cm2 were created in anterior walls of abdominal esophagus. The control group had abdominal esophagus isolated with distal esophageal blunt dissection, and sham group had primary anastomosis. In the test groups, the defects were covered with bilayer polymeric meshes containing FGF (5 μg/2 cm2), or not. All rats were sacrificed for histopathology investigation after 7 or 28 days of operation. The groups are coded as FGF(-)-7th day, FGF(+)-7th day, and FGF(+)-28th day, based on their content and operation day. Highest burst pressures were obtained for FGF(+)-7th day, and FGF(+)-28th day groups (p < 0.005) and decreased inflammation grades were observed. Submucosal and muscular collagen deposition scores were markedly increased in these groups compared to sham and FGF(-)-7th day groups having no FGF (p = 0.002, p = 0.001, respectively). It was proved that FGF loaded bioactive bilayer mesh provided effective repair, reinforcement and tissue healing of esophageal defects.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no conflicts of interest. And there has been no significant financial support for this work that could have influenced its outcomes.
Figures

References
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Miscellaneous

