Prefrontal connectomics: from anatomy to human imaging
- PMID: 34584210
- PMCID: PMC8617085
- DOI: 10.1038/s41386-021-01156-6
Prefrontal connectomics: from anatomy to human imaging
Abstract
The fundamental importance of prefrontal cortical connectivity to information processing and, therefore, disorders of cognition, emotion, and behavior has been recognized for decades. Anatomic tracing studies in animals have formed the basis for delineating the direct monosynaptic connectivity, from cells of origin, through axon trajectories, to synaptic terminals. Advances in neuroimaging combined with network science have taken the lead in developing complex wiring diagrams or connectomes of the human brain. A key question is how well these magnetic resonance imaging (MRI)-derived networks and hubs reflect the anatomic "hard wiring" first proposed to underlie the distribution of information for large-scale network interactions. In this review, we address this challenge by focusing on what is known about monosynaptic prefrontal cortical connections in non-human primates and how this compares to MRI-derived measurements of network organization in humans. First, we outline the anatomic cortical connections and pathways for each prefrontal cortex (PFC) region. We then review the available MRI-based techniques for indirectly measuring structural and functional connectivity, and introduce graph theoretical methods for analysis of hubs, modules, and topologically integrative features of the connectome. Finally, we bring these two approaches together, using specific examples, to demonstrate how monosynaptic connections, demonstrated by tract-tracing studies, can directly inform understanding of the composition of PFC nodes and hubs, and the edges or pathways that connect PFC to cortical and subcortical areas.
© 2021. The Author(s).
Conflict of interest statement
EB is a National Institute of Health Research Senior Investigator and serves on the scientific advisory board of Sosei Heptares and as a consultant for GlaxoSmithKline. The remaining authors declare no competing interests.
Figures
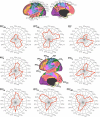
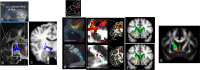
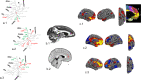
Similar articles
-
Meta-connectomics: human brain network and connectivity meta-analyses.Psychol Med. 2016 Apr;46(5):897-907. doi: 10.1017/S0033291715002895. Epub 2016 Jan 26. Psychol Med. 2016. PMID: 26809184 Free PMC article. Review.
-
Mapping brain-wide excitatory projectome of primate prefrontal cortex at submicron resolution and comparison with diffusion tractography.Elife. 2022 May 20;11:e72534. doi: 10.7554/eLife.72534. Elife. 2022. PMID: 35593765 Free PMC article.
-
Structural Basis of Large-Scale Functional Connectivity in the Mouse.J Neurosci. 2017 Aug 23;37(34):8092-8101. doi: 10.1523/JNEUROSCI.0438-17.2017. Epub 2017 Jul 17. J Neurosci. 2017. PMID: 28716961 Free PMC article.
-
Comparative Primate Connectomics.Brain Behav Evol. 2018;91(3):170-179. doi: 10.1159/000488886. Epub 2018 Aug 10. Brain Behav Evol. 2018. PMID: 30099461 Review.
-
Graph theory analysis identified two hubs that connect sensorimotor and cognitive and cortical and subcortical nociceptive networks in the non-human primate.Neuroimage. 2022 Aug 15;257:119244. doi: 10.1016/j.neuroimage.2022.119244. Epub 2022 May 6. Neuroimage. 2022. PMID: 35533827 Free PMC article.
Cited by
-
Asymmetric signaling across the hierarchy of cytoarchitecture within the human connectome.Sci Adv. 2022 Dec 14;8(50):eadd2185. doi: 10.1126/sciadv.add2185. Epub 2022 Dec 14. Sci Adv. 2022. PMID: 36516263 Free PMC article.
-
Prefrontal-habenular microstructural impairments in human cocaine and heroin addiction.Neuron. 2022 Nov 16;110(22):3820-3832.e4. doi: 10.1016/j.neuron.2022.09.011. Epub 2022 Oct 6. Neuron. 2022. PMID: 36206758 Free PMC article.
-
Mapping dysfunctional circuits in the frontal cortex using deep brain stimulation.Nat Neurosci. 2024 Mar;27(3):573-586. doi: 10.1038/s41593-024-01570-1. Epub 2024 Feb 22. Nat Neurosci. 2024. PMID: 38388734 Free PMC article.
-
Brain laterality evaluated by F-18 fluorodeoxyglucose positron emission computed tomography in autism spectrum disorders.Front Mol Neurosci. 2022 Aug 10;15:901016. doi: 10.3389/fnmol.2022.901016. eCollection 2022. Front Mol Neurosci. 2022. PMID: 36034502 Free PMC article.
-
Right Inferior Frontal Activation During Alcohol-Specific Inhibition Increases With Craving and Predicts Drinking Outcome in Alcohol Use Disorder.Front Psychiatry. 2022 Jul 1;13:909992. doi: 10.3389/fpsyt.2022.909992. eCollection 2022. Front Psychiatry. 2022. PMID: 35845462 Free PMC article.
References
-
- Brodmann K. Vergleichende Lokalisationslehre der Grosshirnrinde in ihren Prinzipien dargestellt auf Grund des Zellenbaues. Leipzig: Barth; 1909. p. x, 324.
-
- Vogt O, Vogt C. Ergebnisse unserer Hirnforschung. J Psychol Neurol. 1919;25:277–462.
-
- Catani M, Ffytche DH. The rises and falls of disconnection syndromes. Brain. 2005;128:2224–39. - PubMed
Publication types
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

