Clinical Manifestations of Cuticular Drusen: Current Perspectives
- PMID: 34584401
- PMCID: PMC8464647
- DOI: 10.2147/OPTH.S272345
Clinical Manifestations of Cuticular Drusen: Current Perspectives
Abstract
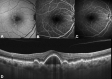
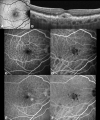
Cuticular drusen are part of the spectrum of age-related macular degeneration (AMD) with particular clinical and multimodal imaging characteristics. This drusen subpopulation shares several high-risk single nucleotide polymorphisms with AMD. Despite this feature, they can manifest at a relatively young age, presenting with a female preponderance. Multimodal imaging is essential for characterizing such lesions, using a combination of color fundus photographs, optical coherence tomography (OCT), fluorescein angiography (FA), and fundus autofluorescence (FAF). The classic starry-sky pattern visible on FA and the typical central hypoautofluorescent lesion with hyperautofluorescent rim on FAF is considered the result of a central retinal pigment epithelium (RPE) erosion from these triangular elevations of the RPE-basal lamina. This finding may also be responsible for the typical choroidal hypertransmission appreciated through OCT. The clinical course of cuticular drusen may be relatively benign at early stages, with small drusen presenting at a young age. However, the presence of clinical phenotypes characterized by diffuse involvement and/or accompanying large drusen in patients older than 60 years may confer a significant risk for either macular neovascularization or geographic atrophy.
Keywords: cuticular drusen; fluorescein angiography; fundus autofluorescence; multimodal imaging; spectral-domain optical coherence tomography.
© 2021 Fragiotta et al.
Conflict of interest statement
The authors report no conflicts of interest in this work.
Figures



References
-
- Gass JDM. Stereoscopic Atlas of Macular Diseases. Diagnosis and Treatment. Mosby CV, 2nd ed. St.Louis; 1977
-
- Farkas TG, Krill AE, Sylvester VM, Archer D. Familial and secondary drusen: histologic and functional correlations. Trans Am Acad Ophthalmol Otolaryngol. 1971;75(2):333–343. - PubMed
Publication types
LinkOut - more resources
Full Text Sources

