Low-temperature crystal structure of 4-chloro-1 H-pyrazole
- PMID: 34584769
- PMCID: PMC8423003
- DOI: 10.1107/S2056989021008604
Low-temperature crystal structure of 4-chloro-1 H-pyrazole
Abstract
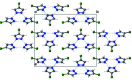
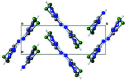
The structure of 4-chloro-1H-pyrazole, C3H3ClN2, at 170 K has ortho-rhom-bic (Pnma) symmetry and is isostructural to its bromo analogue. Data were collected at low temperature since 4-chloro-1H-pyrazole sublimes when subjected to the localized heat produced by X-rays. The structure displays inter-molecular N-H⋯N hydrogen bonding and the packing features a trimeric mol-ecular assembly bis-ected by a mirror plane (m normal to b) running through one chlorine atom, one carbon atom and one N-N bond. The asymmetric unit therefore consists of one and one-half 4-chloro-1H-pyrazole mol-ecules. Thus, the N-H proton is crystallographically disordered over two positions of 50% occupancy each.
Keywords: crystal structure; low temperature; proton disorder; pyrazole.
© Rue and Raptis 2021.
Figures
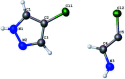
References
-
- Alkorta, I., Elguero, J., Donnadieu, B., Etienne, M., Jaffart, J., Schagen, D. & Limbach, H.-H. (1999). New J. Chem. 23, 1231–1237.
-
- Bruker (2020). APEX3. Bruker AXS Inc., Madison, Wisconsin, USA.
-
- Dolomanov, O. V., Bourhis, L. J., Gildea, R. J., Howard, J. A. K. & Puschmann, H. (2009). J. Appl. Cryst. 42, 339–341.
-
- Farmiloe, S. E., Berdiell, I. C. & Halcrow, M. E. (2019). CSD Communication (deposition No. 1944671). CCDC, Cambridge, England. https://doi.org/10.5517/ccdc.csd.cc238lbb
-
- Foces-Foces, C., Llamas-Saiz, A. L. & Elguero, J. (1999). Z. Kristallogr. 214, 237–241.
LinkOut - more resources
Full Text Sources
Miscellaneous
