Fetal brain issues in congenital heart disease
- PMID: 34584890
- PMCID: PMC8429876
- DOI: 10.21037/tp-20-224
Fetal brain issues in congenital heart disease
Abstract
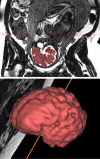
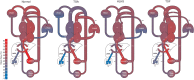
Following the improvements in the clinical management of patients with congenital heart disease (CHD) and their increased survival, neurodevelopmental outcome has become an emerging priority in pediatric cardiology. Large-scale efforts have been made to protect the brain during the postnatal, surgical, and postoperative period; however, the presence of brain immaturity and injury at birth suggests in utero and peripartum disturbances. Over the past decade, there has been considerable interest and investigations on fetal brain growth in the setting of CHD. Advancements in fetal brain imaging have identified abnormal brain development in fetuses with CHD from the macrostructural (brain volumes and cortical folding) down to the microstructural (biochemistry and water diffusivity) scale, with more severe forms of CHD showing worse disturbances and brain abnormalities starting as early as the first trimester. Anomalies in common genetic developmental pathways and diminished cerebral substrate delivery secondary to altered cardiovascular physiology are the forefront hypotheses, but other factors such as impaired placental function and maternal psychological stress have surfaced as important contributors to fetal brain immaturity in CHD. The characterization and timing of fetal brain disturbances and their associated mechanisms are important steps for determining preventative prenatal interventions, which may provide a stronger foundation for the developing brain during childhood.
Keywords: Fetal; brain; congenital heart disease (CHD); imaging.
2021 Translational Pediatrics. All rights reserved.
Conflict of interest statement
Conflicts of Interest: The authors have completed the ICMJE uniform disclosure form (available at http://dx.doi.org/10.21037/tp-20-224). The series “Pre-natal Diagnosis in Congenital Heart Defects” was commissioned by the editorial office without any funding or sponsorship. The authors have no other conflicts of interest to declare.
Figures
Similar articles
-
Association of Maternal Psychological Distress With In Utero Brain Development in Fetuses With Congenital Heart Disease.JAMA Pediatr. 2020 Mar 1;174(3):e195316. doi: 10.1001/jamapediatrics.2019.5316. Epub 2020 Mar 2. JAMA Pediatr. 2020. PMID: 31930365 Free PMC article.
-
Neuroplacentology in congenital heart disease: placental connections to neurodevelopmental outcomes.Pediatr Res. 2022 Mar;91(4):787-794. doi: 10.1038/s41390-021-01521-7. Epub 2021 Apr 16. Pediatr Res. 2022. PMID: 33864014 Free PMC article. Review.
-
Fetal Brain Development in Congenital Heart Disease.Can J Cardiol. 2023 Feb;39(2):115-122. doi: 10.1016/j.cjca.2022.09.020. Epub 2022 Sep 27. Can J Cardiol. 2023. PMID: 36174913 Free PMC article. Review.
-
Prenatal diagnosis of congenital heart defects: echocardiography.Transl Pediatr. 2021 Aug;10(8):2210-2224. doi: 10.21037/tp-20-164. Transl Pediatr. 2021. PMID: 34584892 Free PMC article. Review.
-
[Fetal and genetic aspects of congenital heart disease].Ther Umsch. 2001 Feb;58(2):70-5. doi: 10.1024/0040-5930.58.2.70. Ther Umsch. 2001. PMID: 11234453 Review. German.
Cited by
-
Harnessing the Power of Stem Cell Models to Study Shared Genetic Variants in Congenital Heart Diseases and Neurodevelopmental Disorders.Cells. 2022 Jan 28;11(3):460. doi: 10.3390/cells11030460. Cells. 2022. PMID: 35159270 Free PMC article. Review.
-
Preterm congenital heart disease and neurodevelopment: the importance of looking beyond the initial hospitalization.J Perinatol. 2023 Jul;43(7):958-962. doi: 10.1038/s41372-023-01687-4. Epub 2023 May 13. J Perinatol. 2023. PMID: 37179381 Review.
-
Structural Covariance Networks in the Fetal Brain Reveal Altered Neurodevelopment for Specific Subtypes of Congenital Heart Disease.J Am Heart Assoc. 2024 Nov 5;13(21):e035880. doi: 10.1161/JAHA.124.035880. Epub 2024 Oct 25. J Am Heart Assoc. 2024. PMID: 39450739 Free PMC article.
-
Total and Regional Brain Volumes in Fetuses With Congenital Heart Disease.J Magn Reson Imaging. 2024 Aug;60(2):497-509. doi: 10.1002/jmri.29078. Epub 2023 Oct 17. J Magn Reson Imaging. 2024. PMID: 37846811 Free PMC article.
-
Reference Values for Fetal Cardiac Dimensions, Volumes, Ventricular Function and Left Ventricular Longitudinal Strain Using Doppler Ultrasound Gated Cardiac Magnetic Resonance Imaging in Healthy Third Trimester Fetuses.J Magn Reson Imaging. 2024 Jul;60(1):365-374. doi: 10.1002/jmri.29077. Epub 2023 Oct 19. J Magn Reson Imaging. 2024. PMID: 37855630 Free PMC article.
References
Publication types
LinkOut - more resources
Full Text Sources
Miscellaneous
