Development of alternative gene transfer techniques for ex vivo and in vivo gene therapy in a canine model
- PMID: 34584911
- PMCID: PMC8441024
- DOI: 10.1016/j.reth.2021.08.009
Development of alternative gene transfer techniques for ex vivo and in vivo gene therapy in a canine model
Abstract
Introduction: Gene therapy have recently attracted much attention as a curative therapeutic option for inherited single gene disorders such as hemophilia. Hemophilia is a hereditary bleeding disorder caused by the deficiency of clotting activity of factor VIII (FVIII) or factor IX (FIX), and gene therapy for hemophilia using viral vector have been vigorously investigated worldwide. Toward further advancement of gene therapy for hemophilia, we have previously developed and validated the efficacy of novel two types of gene transfer technologies using a mouse model of hemophilia A. Here we investigated the efficacy and safety of the technologies in canine model. Especially, validations of technical procedures of the gene transfers for dogs were focused.
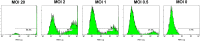
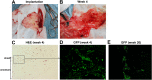
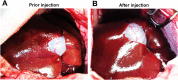
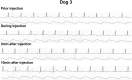
Methods: Green fluorescence protein (GFP) gene were transduced into normal beagle dogs by ex vivo and in vivo gene transfer techniques. For ex vivo gene transfer, blood outgrowth endothelial cells (BOECs) derived from peripheral blood of normal dogs were transduced with GFP gene using lentivirus vector, propagated, fabricated as cell sheets, then implanted onto the omentum of the same dogs. For in vivo gene transfer, normal dogs were subjected to GFP gene transduction with non-viral piggyBac vector by liver-targeted hydrodynamic injections.
Results: No major adverse events were observed during the gene transfers in both gene transfer systems. As for ex vivo gene transfer, histological findings from the omental biopsy performed 4 weeks after implantation revealed the tube formation by implanted GFP-positive BOECs in the sub-adipose tissue layer without any inflammatory findings, and the detected GFP signals were maintained over 6 months. Regarding in vivo gene transfer, analyses of liver biopsy samples revealed more than 90% of liver cells were positive for GFP signals in the injected liver lobes 1 week after gene transfers, then the signals gradually declined overtime.
Conclusions: Two types of gene transfer techniques were successfully applied to a canine model, and the transduced gene expressions persisted for a long term. Toward clinical application for hemophilia patients, practical assessments of therapeutic efficacy of these techniques will need to be performed using a dog model of hemophilia and FVIII (or FIX) gene.
Keywords: BOEC, blood outgrowth endothelial cell; Cell sheet; Dog; FIX, factor IX; FVIII, factor VIII; GFP, green fluorescent protein; Gene therapy; Hemophilia; Hydrodynamic injection.
© 2021 The Japanese Society for Regenerative Medicine. Production and hosting by Elsevier B.V.
Conflict of interest statement
Teruo Okano, Ph.D. is a stockholder of CellSeed Inc. which has licenses for certain cell sheet-related technologies and patents from Tokyo Women's Medical University. Other authors have no interests to declare.
Figures



Similar articles
-
Omental implantation of BOECs in hemophilia dogs results in circulating FVIII antigen and a complex immune response.Blood. 2014 Jun 26;123(26):4045-53. doi: 10.1182/blood-2013-12-545780. Epub 2014 May 14. Blood. 2014. PMID: 24829206
-
Ex vivo gene therapy for hemophilia A that enhances safe delivery and sustained in vivo factor VIII expression from lentivirally engineered endothelial progenitors.Stem Cells. 2007 Oct;25(10):2660-9. doi: 10.1634/stemcells.2006-0699. Epub 2007 Jul 5. Stem Cells. 2007. PMID: 17615271
-
A novel cell-sheet technology that achieves durable factor VIII delivery in a mouse model of hemophilia A.PLoS One. 2013 Dec 16;8(12):e83280. doi: 10.1371/journal.pone.0083280. eCollection 2013. PLoS One. 2013. PMID: 24358271 Free PMC article.
-
Gene therapy for the hemophilias.J Thromb Haemost. 2003 Jul;1(7):1550-8. doi: 10.1046/j.1538-7836.2003.00265.x. J Thromb Haemost. 2003. PMID: 12871290 Review.
-
Hemophilia Gene Therapy: Ready for Prime Time?Hum Gene Ther. 2017 Nov;28(11):1013-1023. doi: 10.1089/hum.2017.116. Epub 2017 Aug 3. Hum Gene Ther. 2017. PMID: 28793786 Review.
Cited by
-
Non-viral and viral delivery systems for hemophilia A therapy: recent development and prospects.Ann Hematol. 2024 May;103(5):1493-1511. doi: 10.1007/s00277-023-05459-0. Epub 2023 Nov 11. Ann Hematol. 2024. PMID: 37951852 Review.
-
Hydrodynamic Delivery: Characteristics, Applications, and Technological Advances.Pharmaceutics. 2023 Mar 31;15(4):1111. doi: 10.3390/pharmaceutics15041111. Pharmaceutics. 2023. PMID: 37111597 Free PMC article. Review.
References
-
- Bolton-Maggs P.H., Pasi K.J. Haemophilias A and B. Lancet. 2003;361:1801–1809. - PubMed
-
- Peters R., Harris T. Advances and innovations in haemophilia treatment. Nat Rev Drug Discov. 2018;17:493–508. - PubMed
-
- Nathwani A.C., Davidoff A.M., Tuddenham E.G.D. Advances in gene therapy for hemophilia. Hum Gene Ther. 2017;28:1004–1012. - PubMed
LinkOut - more resources
Full Text Sources
Research Materials

