Trace Hydrogen Sulfide Sensing Inspired by Polyoxometalate-Mediated Aerobic Oxidation
- PMID: 34584959
- PMCID: PMC8461779
- DOI: 10.1021/acscentsci.1c00746
Trace Hydrogen Sulfide Sensing Inspired by Polyoxometalate-Mediated Aerobic Oxidation
Abstract
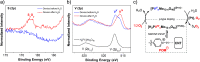
A high-performance chemiresistive gas sensor is described for the detection of hydrogen sulfide (H2S), an acutely toxic and corrosive gas. The chemiresistor operates at room temperature with low power requirements potentially suitable for wearable sensors or for rapid in-field detection of H2S in settings such as pipelines and wastewater treatment plants. Specifically, we report chemiresistors based on single-walled carbon nanotubes (SWCNTs) containing highly oxidizing platinum-polyoxometalate (Pt-POM) selectors. We show that by tuning the vanadium content and thereby the oxidation reactivity of the constituent POMs, an efficient chemiresistive sensor is obtained that is proposed to operate by modulating CNT doping during aerobic H2S oxidation. The sensor shows exceptional sensitivity to trace H2S in air with a ppb-level detection limit, multimonth stability under ambient conditions, and high selectivity for H2S over a wide range of interferants, including thiols, thioethers, and thiophene. Finally, we demonstrate that the robust sensing material can be used to fabricate flexible devices by covalently immobilizing the SWCNT-P4VP network onto a polyimide substrate, further extending the potentially broad utility of the chemiresistors. The strategy presented herein highlights the applicability of concepts in molecular aerobic oxidation catalysis to the development of low-cost analyte detection technologies.
© 2021 The Authors. Published by American Chemical Society.
Conflict of interest statement
The authors declare the following competing financial interest(s): A patent has been filed on this method.
Figures






References
-
- Hammer G.; Lübcke T.; Kettner R.; Pillarella M. R.; Recknagel H.; Commichau A.; Neumann H.-J.; Paczynska-Lahme B. Natural Gas. In Ullmann’s Encyclopedia of Industrial Chemistry; Wiley-VCH: 2006.
-
-
NIOSH Pocket Guide to Chemical Hazards. “#0337”. National Institute for Occupational Safety and Health (NIOSH).
-
LinkOut - more resources
Full Text Sources
Other Literature Sources

