Bioorthogonal chemistry
- PMID: 34585143
- PMCID: PMC8469592
- DOI: 10.1038/s43586-021-00028-z
Bioorthogonal chemistry
Abstract
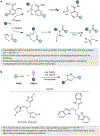
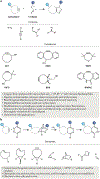
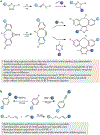
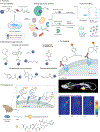
Bioorthogonal chemistry represents a class of high-yielding chemical reactions that proceed rapidly and selectively in biological environments without side reactions towards endogenous functional groups. Rooted in the principles of physical organic chemistry, bioorthogonal reactions are intrinsically selective transformations not commonly found in biology. Key reactions include native chemical ligation and the Staudinger ligation, copper-catalysed azide-alkyne cycloaddition, strain-promoted [3 + 2] reactions, tetrazine ligation, metal-catalysed coupling reactions, oxime and hydrazone ligations as well as photoinducible bioorthogonal reactions. Bioorthogonal chemistry has significant overlap with the broader field of 'click chemistry' - high-yielding reactions that are wide in scope and simple to perform, as recently exemplified by sulfuryl fluoride exchange chemistry. The underlying mechanisms of these transformations and their optimal conditions are described in this Primer, followed by discussion of how bioorthogonal chemistry has become essential to the fields of biomedical imaging, medicinal chemistry, protein synthesis, polymer science, materials science and surface science. The applications of bioorthogonal chemistry are diverse and include genetic code expansion and metabolic engineering, drug target identification, antibody-drug conjugation and drug delivery. This Primer describes standards for reproducibility and data deposition, outlines how current limitations are driving new research directions and discusses new opportunities for applying bioorthogonal chemistry to emerging problems in biology and biomedicine.
Conflict of interest statement
Competing interests W.L. and C.W.a.E. are employees of Pfizer Inc. M.S.R. is an employee and shareholder of Tagworks Pharmaceuticals. S.L.S., D.A.B., R.H., S.S.N., M.X., M.G.F., K.L., Q.L., J.P.P., J.A.P. and J.M.F. declare no competing interests.
Figures



References
-
- Nienhaus GU The green fluorescent protein: a key tool to study chemical processes in living cells. Angew. Chem. Int. Ed 47, 8992–8994 (2008). - PubMed
-
- Winter G & Milstein C Man-made antibodies. Nature 349, 293–299 (1991). - PubMed
-
- Lang K & Chin JW Bioorthogonal reactions for labeling proteins. ACS Chem. Biol 9, 16–20 (2014). - PubMed
-
- Kolb HC, Finn MG & Sharpless KB Click chemistry: diverse chemical function from a few good reactions. Angew. Chem. Int. Ed 40, 2004–2021 (2001). - PubMed
-
This seminal review article outlines the concept and requirements of click chemistry.
-
- Dong J, Krasnova L, Finn MG & Sharpless KB Sulfur(VI) fluoride exchange (SuFEx): another good reaction for click chemistry. Angew. Chem. Int. Ed 53, 9430–9448 (2014). - PubMed
-
This article presents an initial description of SuFEx chemistry as an efficient biocompatible click chemistry tool.
Grants and funding
LinkOut - more resources
Full Text Sources
Other Literature Sources
