Computational simulation-derived hemodynamic and biomechanical properties of the pulmonary arterial tree early in the course of ventricular septal defects
- PMID: 34585299
- PMCID: PMC9704055
- DOI: 10.1007/s10237-021-01519-4
Computational simulation-derived hemodynamic and biomechanical properties of the pulmonary arterial tree early in the course of ventricular septal defects
Abstract
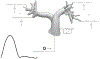
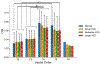
Untreated ventricular septal defects (VSDs) can lead to pulmonary arterial hypertension (PAH) characterized by elevated pulmonary artery (PA) pressure and vascular remodeling, known as PAH associated with congenital heart disease (PAH-CHD). Though previous studies have investigated hemodynamic effects on vascular mechanobiology in late-stage PAH, hemodynamics leading to PAH-CHD initiation have not been fully quantified. We hypothesize that abnormal hemodynamics from left-to-right shunting in early stage VSDs affects PA biomechanical properties leading to PAH initiation. To model PA hemodynamics in healthy, small, moderate, and large VSD conditions prior to the onset of vascular remodeling, computational fluid dynamics simulations were performed using a 3D finite element model of a healthy 1-year-old's proximal PAs and a body-surface-area-scaled 0D distal PA tree. VSD conditions were modeled with increased pulmonary blood flow to represent degrees of left-to-right shunting. In the proximal PAs, pressure, flow, strain, and wall shear stress (WSS) increased with increasing VSD size; oscillatory shear index decreased with increasing VSD size in the larger PA vessels. WSS was higher in smaller diameter vessels and increased with VSD size, with the large VSD condition exhibiting WSS >100 dyn/cm[Formula: see text], well above values typically used to study dysfunctional mechanotransduction pathways in PAH. This study is the first to estimate hemodynamic and biomechanical metrics in the entire pediatric PA tree with VSD severity at the stage leading to PAH initiation and has implications for future studies assessing effects of abnormal mechanical stimuli on endothelial cells and vascular wall mechanics that occur during PAH-CHD initiation and progression.
Keywords: Computational cardiovascular biomechanics; Congenital heart defect; Pulmonary arterial hypertension; Pulmonary arterial tree; Ventricular septal defect.
© 2021. The Author(s), under exclusive licence to Springer-Verlag GmbH Germany, part of Springer Nature.
Figures

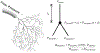


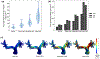
References
-
- Al-Nassri S, Unny T (1981) Developing laminar flow in the inlet length of a smooth pipe. Appl Sci Res 36:313–332. 10.1007/BF00411891 - DOI
-
- Barker AJ, Roldán-Alzate A, Entezari P, Shah SJ, Chesler NC, Wieben O, Markl M, François CJ (2015) Four-dimensional flow assessment of pulmonary artery flow and wall shear stress in adult pulmonary arterial hypertension: results from two institutions. Magn Reson Med 73(5):1904–1913. 10.1002/mrm.25326 - DOI - PMC - PubMed
MeSH terms
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials

