CAR-T therapy: Prospects in targeting cancer stem cells
- PMID: 34585512
- PMCID: PMC8572776
- DOI: 10.1111/jcmm.16939
CAR-T therapy: Prospects in targeting cancer stem cells
Abstract
Cancer stem cells (CSCs), a group of tumour cells with stem cell characteristics, have the ability of self-renewal, multi-lineage differentiation and tumour formation. Since CSCs are resistant to conventional radiotherapy and chemotherapy, their existence may be one of the root causes of cancer treatment failure and tumour progression. The elimination of CSCs may be effective for eventual tumour eradication. Because of the good therapeutic effects without major histocompatibility complex (MHC) restriction and the unique characteristics of CSCs, chimeric antigen receptor T-cell (CAR-T) therapy is expected to be an important method to eliminate CSCs. In this review, we have discussed the feasibility of CSCs-targeted CAR-T therapy for cancer treatment, summarized current research and clinical trials of targeting CSCs with CAR-T cells and forecasted the challenges and future direction from the perspectives of toxicity, persistence and potency, trafficking, infiltration, immunosuppressive tumour microenvironment, and tumour heterogeneity.
Keywords: CAR-T; cancer stem cells; immunotherapy; targeted therapy.
© 2021 The Authors. Journal of Cellular and Molecular Medicine published by Foundation for Cellular and Molecular Medicine and John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of interest relevant to this article was not reported.
Figures
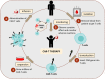
References
-
- Cohnheim J. Ueber entzündung und eiterung. Arch Pathol Anat Physiol Klin Med. 1867;40(1):1‐79.
-
- Clara JA, Monge C, Yang Y, Takebe N. Targeting signalling pathways and the immune microenvironment of cancer stem cells — a clinical update. Nat Rev Clin Oncol. 2020;17(4):204‐232. - PubMed
-
- Quaglino E, Conti L, Cavallo F. Breast cancer stem cell antigens as targets for immunotherapy. Semin Immunol. 2020;47:101386. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Research Materials

