Photoredox-Catalyzed C-H Functionalization Reactions
- PMID: 34585909
- PMCID: PMC8939264
- DOI: 10.1021/acs.chemrev.1c00311
Photoredox-Catalyzed C-H Functionalization Reactions
Abstract
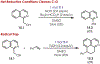
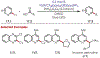
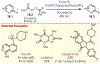
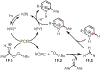
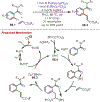
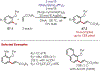
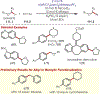
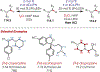
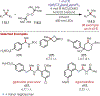
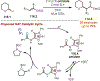
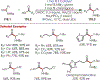
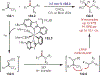
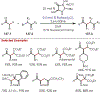
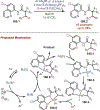
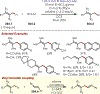
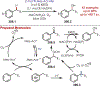
The fields of C-H functionalization and photoredox catalysis have garnered enormous interest and utility in the past several decades. Many different scientific disciplines have relied on C-H functionalization and photoredox strategies including natural product synthesis, drug discovery, radiolabeling, bioconjugation, materials, and fine chemical synthesis. In this Review, we highlight the use of photoredox catalysis in C-H functionalization reactions. We separate the review into inorganic/organometallic photoredox catalysts and organic-based photoredox catalytic systems. Further subdivision by reaction class─either sp2 or sp3 C-H functionalization─lends perspective and tactical strategies for use of these methods in synthetic applications.
Conflict of interest statement
The authors declare no competing financial interest.
Figures
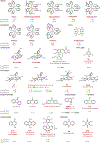
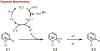
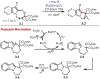

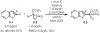
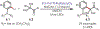
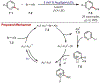
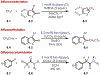
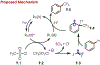
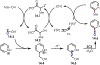
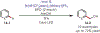
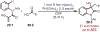
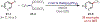

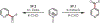
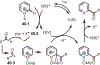


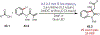
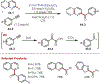
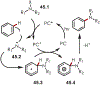

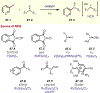

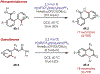
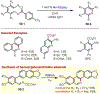



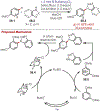
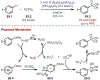
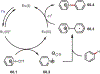
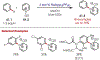
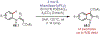
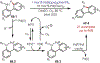
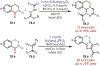
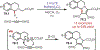

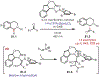
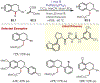
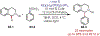


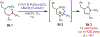
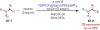
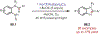
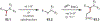
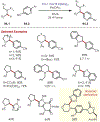

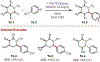
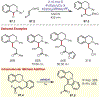

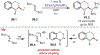
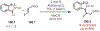

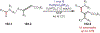

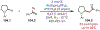
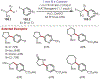
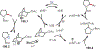


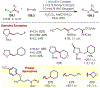
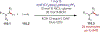
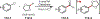

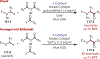

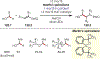



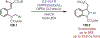
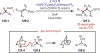
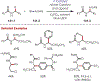
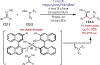

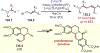





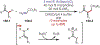
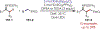
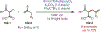
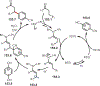
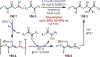
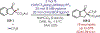
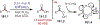



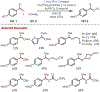

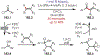
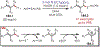
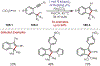
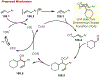
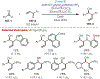
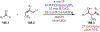


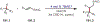
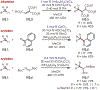

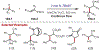
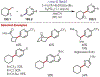

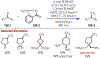






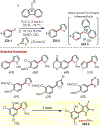
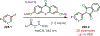
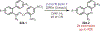
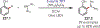
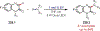
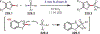
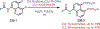
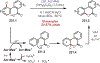
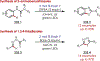
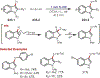
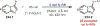
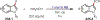

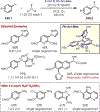
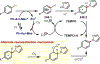
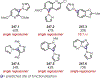

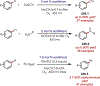
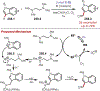
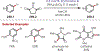
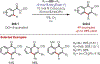
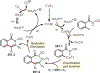




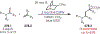
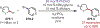
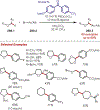
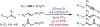
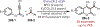
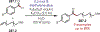
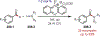

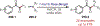
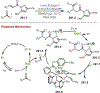
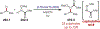
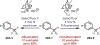
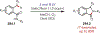

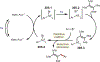
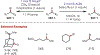

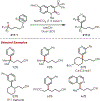
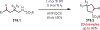
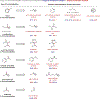
Similar articles
-
Synthetic and Mechanistic Implications of Chlorine Photoelimination in Nickel/Photoredox C(sp3)-H Cross-Coupling.Acc Chem Res. 2021 Feb 16;54(4):988-1000. doi: 10.1021/acs.accounts.0c00694. Epub 2021 Jan 29. Acc Chem Res. 2021. PMID: 33511841 Free PMC article.
-
Photoredox catalysis in nickel-catalyzed C-H functionalization.Beilstein J Org Chem. 2021 Aug 31;17:2209-2259. doi: 10.3762/bjoc.17.143. eCollection 2021. Beilstein J Org Chem. 2021. PMID: 34621388 Free PMC article. Review.
-
Recent developments in photoredox-catalyzed remote ortho and para C-H bond functionalizations.Beilstein J Org Chem. 2020 Feb 26;16:248-280. doi: 10.3762/bjoc.16.26. eCollection 2020. Beilstein J Org Chem. 2020. PMID: 32180843 Free PMC article. Review.
-
Merging Visible Light Photoredox and Gold Catalysis.Acc Chem Res. 2016 Oct 18;49(10):2261-2272. doi: 10.1021/acs.accounts.6b00351. Epub 2016 Sep 9. Acc Chem Res. 2016. PMID: 27610939
-
Exploration of C-H Transformations of Aldehyde Hydrazones: Radical Strategies and Beyond.Acc Chem Res. 2018 Feb 20;51(2):484-495. doi: 10.1021/acs.accounts.7b00565. Epub 2018 Jan 23. Acc Chem Res. 2018. PMID: 29359909
Cited by
-
Photoinduced cerium-catalyzed C-H acylation of unactivated alkanes.Chem Sci. 2023 Nov 25;15(1):154-159. doi: 10.1039/d3sc05162e. eCollection 2023 Dec 20. Chem Sci. 2023. PMID: 38131082 Free PMC article.
-
Electrochemical radical cation aza-Wacker cyclizations.Beilstein J Org Chem. 2024 Aug 5;20:1900-1905. doi: 10.3762/bjoc.20.165. eCollection 2024. Beilstein J Org Chem. 2024. PMID: 39135656 Free PMC article.
-
Photocatalytic regioselective C-H bond functionalizations in arenes.Chem Sci. 2024 Dec 9;16(3):1041-1070. doi: 10.1039/d4sc07491b. eCollection 2025 Jan 15. Chem Sci. 2024. PMID: 39691465 Free PMC article. Review.
-
Nile Red-Based Covalent Organic Framework as a Photocatalyst for C-H Bond Functionalization.ACS Catal. 2025 Jun 6;15(12):10736-10745. doi: 10.1021/acscatal.5c02173. eCollection 2025 Jun 20. ACS Catal. 2025. PMID: 40568222 Free PMC article.
-
Expanding Perchlorate Use for C-H Oxidative Transformations: A Tandem Photo- and Iron-Catalytic Strategy.Organometallics. 2025 Apr 14;44(7):777-782. doi: 10.1021/acs.organomet.4c00516. Epub 2025 Mar 14. Organometallics. 2025. PMID: 40654525
References
-
- Arndtsen BA; Bergman RG; Mobley TA; Peterson TH Selective Intermolecular Carbon-Hydrogen Bond Activation by Synthetic Metal Complexes in Homogeneous Solution. Acc. Chem. Res 1995, 28, 154–162.
-
- Alberico D; Scott ME; Lautens M Aryl-Aryl Bond Formation by Transition-Metal-Catalyzed Direct Arylation. Chem. Rev 2007, 107, 174–238. - PubMed
-
- Mkhalid IAI; Barnard JH; Marder TB; Murphy JM; Hartwig JF C-H Activation for the Construction of C-B Bonds. Chem. Rev 2010, 110, 890–931. - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

