Helicobacter pylori and Epstein-Barr Virus Coinfection Stimulates Aggressiveness in Gastric Cancer through the Regulation of Gankyrin
- PMID: 34585958
- PMCID: PMC8550222
- DOI: 10.1128/mSphere.00751-21
Helicobacter pylori and Epstein-Barr Virus Coinfection Stimulates Aggressiveness in Gastric Cancer through the Regulation of Gankyrin
Abstract
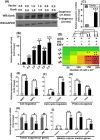
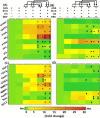
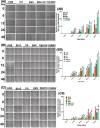
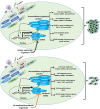
Persistent coinfection with Helicobacter pylori and Epstein-Barr virus (EBV) promotes aggressive gastric carcinoma (GC). The molecular mechanisms underlying the aggressiveness in H. pylori and EBV-mediated GC are not well characterized. We investigated the molecular mechanism involved in H. pylori- and EBV-driven proliferation of gastric epithelial cells. Results showed that the coinfection is significantly more advantageous to the pathogens as coinfection creates a microenvironment favorable to higher pathogen-associated gene expression. The EBV latent genes ebna1 and ebna3c are highly expressed in the coinfection compared to lone EBV infection at 12 and 24 h. The H. pylori-associated genes 16S rRNA, cagA, and babA were also highly expressed during coinfection compared to H. pylori alone. In addition, upregulation of gankyrin, which is a small oncoprotein, modulates various cell signaling pathways, leading to oncogenesis. Notably, the knockdown of gankyrin decreased the cancer properties of gastric epithelial cells. Gankyrin showed a similar expression pattern as that of ebna3c at both transcript and protein levels, suggesting a possible correlation. Further, EBV and H. pylori created a microenvironment that induced cell transformation and oncogenesis through dysregulation of the cell cycle regulatory (ccnd1, dapk3, pcna, and akt), GC marker (abl1, tff-2, and cdx2), cell migration (mmp3 and mmp7), DNA response (pRB, pten, and p53), and antiapoptotic (bcl2) genes in infected gastric epithelial cells through gankyrin. Our study provides a new insight into the interplay of two oncogenic agents (H. pylori and EBV) that leads to an enhanced carcinogenic activity in gastric epithelial cells through overexpression of gankyrin. IMPORTANCE In the present study, we evaluated the synergistic effects of EBV and H. pylori infection on gastric epithelial cells in various coinfection models. These coinfection models were among the first to depict the exposures of gastric epithelial cells to EBV followed by H. pylori; however, coinfection models exist that narrated the scenario upon exposure to H. pylori followed by that to EBV. We determined that a coinfection by EBV and H. pylori enhanced the expression of oncogenic protein gankyrin. The interplay between EBV and H. pylori promoted the oncogenic properties of AGS cells like elevated focus formation, cell migration, and cell proliferation through gankyrin. EBV and H. pylori mediated an enhanced expression of gankyrin, which further dysregulated cancer-associated genes such as cell migratory, tumor suppressor, DNA damage response, and proapoptotic genes.
Keywords: Epstein-Barr virus; Helicobacter pylori; coinfection; gankyrin; gastric cancer.
Figures
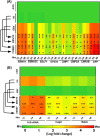

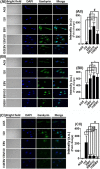
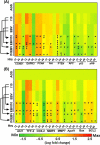



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Research Materials
Miscellaneous

