Candida albicans ENT2 Contributes to Efficient Endocytosis, Cell Wall Integrity, Filamentation, and Virulence
- PMID: 34585966
- PMCID: PMC8550084
- DOI: 10.1128/mSphere.00707-21
Candida albicans ENT2 Contributes to Efficient Endocytosis, Cell Wall Integrity, Filamentation, and Virulence
Abstract
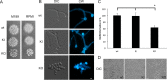
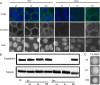
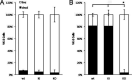
Epsins play a pivotal role in the formation of endocytic vesicles and potentially provide a linkage between endocytic and other trafficking pathways. We identified a Candida albicans epsin, ENT2, that bears homology to the Saccharomyces cerevisiae early endocytosis genes ENT1 and ENT2 and studied its functions by a reverse genetic approach utilizing CRISPR-Cas9-mediated gene deletion. The C. albicans ent2Δ/Δ null mutant displayed cell wall defects and altered antifungal drug sensitivity. To define the role of C. albicans ENT2 in endocytosis, we performed assays with the lipophilic dye FM4-64 that revealed greatly reduced uptake in the ent2Δ/Δ mutant. Next, we showed that the C. albicans ent2Δ/Δ mutant was unable to form hyphae and biofilms. Assays for virulence properties in an in vitro keratinocyte infection model demonstrated reduced damage of mammalian adhesion zippers and host cell death from the ent2Δ/Δ mutant. We conclude that C. albicans ENT2 has a role in efficient endocytosis, a process that is required for maintaining cell wall integrity, hyphal formation, and virulence-defining traits. IMPORTANCE The opportunistic fungal pathogen Candida albicans is an important cause of invasive infections in hospitalized patients and a source of considerable morbidity and mortality. Despite its clinical importance, we still need to improve our ability to diagnose and treat this common pathogen. In order to support these advancements, a greater understanding of the biology of C. albicans is needed. In these studies, we are focused on the fundamental biological process of endocytosis, of which little is directly known in C. albicans. In addition to studying the function of a key gene in this process, we are examining the role of endocytosis in the virulence-related processes of filamentation, biofilm formation, and tissue invasion. These studies will provide greater insight into the role of endocytosis in causing invasive fungal infections.
Keywords: Candida albicans; biofilm; endocytosis; filamentation; membrane trafficking; pathogenesis; secretion.
Figures





References
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
