Sugar-Phosphate Toxicities
- PMID: 34585982
- PMCID: PMC8483676
- DOI: 10.1128/MMBR.00123-21
Sugar-Phosphate Toxicities
Abstract
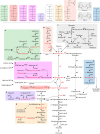
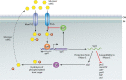
Accumulation of phosphorylated intermediates during cellular metabolism can have wide-ranging toxic effects on many organisms, including humans and the pathogens that infect them. These toxicities can be induced by feeding an upstream metabolite (a sugar, for instance) while simultaneously blocking the appropriate metabolic pathway with either a mutation or an enzyme inhibitor. Here, we survey the toxicities that can arise in the metabolism of glucose, galactose, fructose, fructose-asparagine, glycerol, trehalose, maltose, mannose, mannitol, arabinose, and rhamnose. Select enzymes in these metabolic pathways may serve as novel therapeutic targets. Some are conserved broadly among prokaryotes and eukaryotes (e.g., glucose and galactose) and are therefore unlikely to be viable drug targets. However, others are found only in bacteria (e.g., fructose-asparagine, rhamnose, and arabinose), and one is found in fungi but not in humans (trehalose). We discuss what is known about the mechanisms of toxicity and how resistance is achieved in order to identify the prospects and challenges associated with targeted exploitation of these pervasive metabolic vulnerabilities.
Keywords: antimicrobials; drug targets; fructose-asparagine; sugar phosphate; toxicity.
Figures




References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

