Genetic Networks That Govern Sexual Reproduction in the Pezizomycotina
- PMID: 34585983
- PMCID: PMC8485983
- DOI: 10.1128/MMBR.00020-21
Genetic Networks That Govern Sexual Reproduction in the Pezizomycotina
Abstract
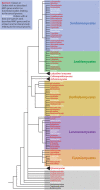
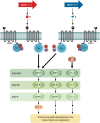
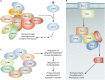
Sexual development in filamentous fungi is a complex process that relies on the precise control of and interaction between a variety of genetic networks and pathways. The mating-type (MAT) genes are the master regulators of this process and typically act as transcription factors, which control the expression of genes involved at all stages of the sexual cycle. In many fungi, the sexual cycle typically begins when the mating pheromones of one mating type are recognized by a compatible partner, followed by physical interaction and fertilization. Subsequently, highly specialized sexual structures are formed, within which the sexual spores develop after rounds of meiosis and mitosis. These spores are then released and germinate, forming new individuals that initiate new cycles of growth. This review provides an overview of the known genetic networks and pathways that are involved in each major stage of the sexual cycle in filamentous ascomycete fungi.
Keywords: MAT genes; Pezizomycotina; fertility; pheromones; sexual reproduction.
Figures



References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

