PGRMC1 Exerts Its Function of Anti-Influenza Virus in the Central Nervous System
- PMID: 34585989
- PMCID: PMC8557870
- DOI: 10.1128/Spectrum.00734-21
PGRMC1 Exerts Its Function of Anti-Influenza Virus in the Central Nervous System
Abstract
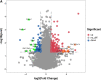
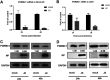
The influenza A virus (IAV) infection is usually restricted to the respiratory tract and only rarely enters the central nervous system (CNS) and causes neurological symptoms. However, the roles of host factors involved in IAV infection in the CNS remain largely undetermined. Therefore, we aimed to characterize the host responses to IAV infection in the brain. We isolated a strain of IAV H5N6, which is neurotoxic and highly pathogenic to mice. High-throughput RNA sequencing (RNA-seq) revealed 240 differentially expressed genes in IAV-infected brains. Among the significantly downregulated genes, we focused on the gene encoding progesterone receptor membrane component-1 (PGRMC1) and observed that IAV H5N6 infection clearly inhibited PGRMC1 in both neuroblastoma and glioma cells. Furthermore, treatment with AG205, a PGRMC1-specific inhibitor, or PGRMC1 knockout promoted H5N6 multiplication in vitro, while overexpression of PGRMC1 resulted in opposite effects. Furthermore, AG205 treatment or PGRMC1 knockout significantly inhibited the retinoic acid-inducible gene I (RIG-I)-mediated interferon beta (IFN-β) signaling pathway and reduced the levels of several antiviral proteins (Mx1 and ISG15). In addition, PGRMC1-mediated regulation of IFN signaling relied on inhibition of the expression and ubiquitination of RIG-I. The loss of PGRMC1 leads to an increased susceptibility of mice (brain and lung) to influenza A virus infection. Conclusively, our results show for the first time that IAV H5N6 downregulates PGRMC1 expression to contribute to virus proliferation by inhibiting RIG-I-mediated IFN-β production in the brain. These findings may offer new insights regarding the interplay between IAV and host factors that may impact IAV pathogenicity in the brain. IMPORTANCE Central nervous system (CNS) disease is one of the most common extra-respiratory tract complications of influenza A virus (IAV) infections. However, there is still little knowledge about IAV regulating host responses in brain. In this study, we identified progesterone receptor membrane component-1 (PGRMC1) as a novel host factor involved in the replication and propagation of IAV H5N6 in the host brain. We also observed that PGRMC1 antagonism was required for viral evasion from the host immune response during IAV infection via inhibition of the retinoic acid-inducible gene I (RIG-I)-mediated interferon beta (IFN-β) signaling pathway and downstream antiviral gene expression. This study revealed a newly identified regulatory mechanism used by IAV H5N6 to ensure its life cycle in the CNS.
Keywords: CNS; H5N6 virus; PGRMC1; RIG-I; virus replication.
Figures






Similar articles
-
CaMKII-dependent non-canonical RIG-I pathway promotes influenza virus propagation in the acute-phase of infection.mBio. 2025 Jan 8;16(1):e0008724. doi: 10.1128/mbio.00087-24. Epub 2024 Nov 27. mBio. 2025. PMID: 39601535 Free PMC article.
-
Influenza A virus-induced degradation of eukaryotic translation initiation factor 4B contributes to viral replication by suppressing IFITM3 protein expression.J Virol. 2014 Aug;88(15):8375-85. doi: 10.1128/JVI.00126-14. Epub 2014 May 14. J Virol. 2014. PMID: 24829357 Free PMC article.
-
IRF5 Promotes Influenza Virus-Induced Inflammatory Responses in Human Induced Pluripotent Stem Cell-Derived Myeloid Cells and Murine Models.J Virol. 2020 Apr 16;94(9):e00121-20. doi: 10.1128/JVI.00121-20. Print 2020 Apr 16. J Virol. 2020. PMID: 32075938 Free PMC article.
-
Modulation of Innate Immune Responses by the Influenza A NS1 and PA-X Proteins.Viruses. 2018 Dec 12;10(12):708. doi: 10.3390/v10120708. Viruses. 2018. PMID: 30545063 Free PMC article. Review.
-
Host Non-Coding RNA Regulates Influenza A Virus Replication.Viruses. 2021 Dec 29;14(1):51. doi: 10.3390/v14010051. Viruses. 2021. PMID: 35062254 Free PMC article. Review.
Cited by
-
Interferon-induced protein ISG15 in the central nervous system, quo vadis?FEBS Lett. 2025 May 12:10.1002/1873-3468.70063. doi: 10.1002/1873-3468.70063. Online ahead of print. FEBS Lett. 2025. PMID: 40353372 Review.
-
Influenza A Virus PB1-F2 Induces Affective Disorder by Interfering Synaptic Plasticity in Hippocampal Dentate Gyrus.Mol Neurobiol. 2024 Oct;61(10):8293-8306. doi: 10.1007/s12035-024-04107-6. Epub 2024 Mar 15. Mol Neurobiol. 2024. PMID: 38488981
-
Host Innate Antiviral Response to Influenza A Virus Infection: From Viral Sensing to Antagonism and Escape.Pathogens. 2024 Jul 3;13(7):561. doi: 10.3390/pathogens13070561. Pathogens. 2024. PMID: 39057788 Free PMC article. Review.
-
Influenza Virus Host Restriction Factors: The ISGs and Non-ISGs.Pathogens. 2024 Jan 29;13(2):127. doi: 10.3390/pathogens13020127. Pathogens. 2024. PMID: 38392865 Free PMC article. Review.
References
-
- Bi Y, Chen Q, Wang Q, Chen J, Jin T, Wong G, Quan C, Liu J, Wu J, Yin R, Zhao L, Li M, Ding Z, Zou R, Xu W, Li H, Wang H, Tian K, Fu G, Huang Y, Shestopalov A, Li S, Xu B, Yu H, Luo T, Lu L, Xu X, Luo Y, Liu Y, Shi W, Liu D, Gao GF. 2016. Genesis, evolution and prevalence of H5N6 avian influenza viruses in China. Cell Host Microbe 20:810–821. doi:10.1016/j.chom.2016.10.022. - DOI - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Molecular Biology Databases
Research Materials
Miscellaneous

