mRNA vaccination in people over 80 years of age induces strong humoral immune responses against SARS-CoV-2 with cross neutralization of P.1 Brazilian variant
- PMID: 34586068
- PMCID: PMC8500710
- DOI: 10.7554/eLife.69375
mRNA vaccination in people over 80 years of age induces strong humoral immune responses against SARS-CoV-2 with cross neutralization of P.1 Brazilian variant
Abstract
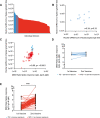
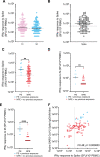
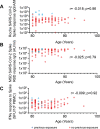
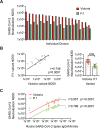
Age is the major risk factor for mortality after SARS-CoV-2 infection and older people have received priority consideration for COVID-19 vaccination. However, vaccine responses are often suboptimal in this age group and few people over the age of 80 years were included in vaccine registration trials. We determined the serological and cellular response to spike protein in 100 people aged 80-96 years at 2 weeks after the second vaccination with the Pfizer BNT162b2 mRNA vaccine. Antibody responses were seen in every donor with high titers in 98%. Spike-specific cellular immune responses were detectable in only 63% and correlated with humoral response. Previous SARS-CoV-2 infection substantially increased antibody responses after one vaccine and antibody and cellular responses remained 28-fold and 3-fold higher, respectively, after dual vaccination. Post-vaccine sera mediated strong neutralization of live Victoria infection and although neutralization titers were reduced 14-fold against the P.1 variant first discovered in Brazil they remained largely effective. These data demonstrate that the mRNA vaccine platform delivers strong humoral immunity in people up to 96 years of age and retains broad efficacy against the P.1 variant of concern.
Keywords: COVID-19; human; immunosenescence; infectious disease; microbiology; vaccination.
© 2021, Parry et al.
Conflict of interest statement
HP, GT, RB, SF, CS, PS, CB, KH, KB, GA, SC, SL, EC, NC, KB, EP, CR, AO, RW, SD, BH, AR, JZ, PM No competing interests declared, AM is affiliated with Oxford Immunotec Ltd. The author has no financial interests to declare.
Figures

References
-
- Abu Jabal K, Ben-Amram H, Beiruti K, Batheesh Y, Sussan C, Zarka S, Edelstein M. Impact of age, ethnicity, sex and prior infection status on immunogenicity following a single dose of the BNT162B2 mrna covid-19 vaccine: Real-world evidence from healthcare workers, Israel, December 2020 to January 2021. Euro Surveillance. 2021;26:2100096. doi: 10.2807/1560-7917.ES.2021.26.6.2100096. - DOI - PMC - PubMed
-
- Bernal JL, Andrews N, Gower C. Early Effectiveness of COVID-19 Vaccination with Bnt162b2 Mrna Vaccine and Chadox1 Adenovirus Vector Vaccine on Symptomatic Disease, Hospitalisations and Mortality in Older Adults in England. medRxiv. 2021 doi: 10.1101/2021.03.01.21252652. - DOI
-
- Chen RE, Zhang X, Case JB, Winkler ES, Liu Y, VanBlargan LA, Liu J, Errico JM, Xie X, Suryadevara N, Gilchuk P, Zost SJ, Tahan S, Droit L, Turner JS, Kim W, Schmitz AJ, Thapa M, Wang D, Boon ACM, Presti RM, O’Halloran JA, Kim AHJ, Deepak P, Pinto D, Fremont DH, Crowe JE, Jr, Corti D, Virgin HW, Ellebedy AH, Shi P-Y, Diamond MS. Resistance of SARS-COV-2 variants to neutralization by monoclonal and serum-derived polyclonal antibodies. Nature Medicine. 2021;27:717–726. doi: 10.1038/s41591-021-01294-w. - DOI - PMC - PubMed
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

