Multidrug efflux transporter ABCG2: expression and regulation
- PMID: 34586444
- PMCID: PMC11072723
- DOI: 10.1007/s00018-021-03901-y
Multidrug efflux transporter ABCG2: expression and regulation
Abstract
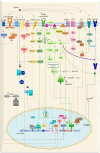
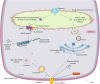
The adenosine triphosphate (ATP)-binding cassette efflux transporter G2 (ABCG2) was originally discovered in a multidrug-resistant breast cancer cell line. Studies in the past have expanded the understanding of its role in physiology, disease pathology and drug resistance. With a widely distributed expression across different cell types, ABCG2 plays a central role in ATP-dependent efflux of a vast range of endogenous and exogenous molecules, thereby maintaining cellular homeostasis and providing tissue protection against xenobiotic insults. However, ABCG2 expression is subjected to alterations under various pathophysiological conditions such as inflammation, infection, tissue injury, disease pathology and in response to xenobiotics and endobiotics. These changes may interfere with the bioavailability of therapeutic substrate drugs conferring drug resistance and in certain cases worsen the pathophysiological state aggravating its severity. Considering the crucial role of ABCG2 in normal physiology, therapeutic interventions directly targeting the transporter function may produce serious side effects. Therefore, modulation of transporter regulation instead of inhibiting the transporter itself will allow subtle changes in ABCG2 activity. This requires a thorough comprehension of diverse factors and complex signaling pathways (Kinases, Wnt/β-catenin, Sonic hedgehog) operating at multiple regulatory levels dictating ABCG2 expression and activity. This review features a background on the physiological role of transporter, factors that modulate ABCG2 levels and highlights various signaling pathways, molecular mechanisms and genetic polymorphisms in ABCG2 regulation. This understanding will aid in identifying potential molecular targets for therapeutic interventions to overcome ABCG2-mediated multidrug resistance (MDR) and to manage ABCG2-related pathophysiology.
Keywords: ABCG2; ATP-binding cassette transporter; Drug resistance; Efflux transporter; Regulation; Signaling pathways.
© 2021. The Author(s), under exclusive licence to Springer Nature Switzerland AG.
Conflict of interest statement
The authors declare that they have no conflict of interest.
Figures
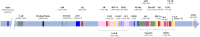

References
Publication types
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources
Miscellaneous

