Iron oxide nanoparticles augment the intercellular mitochondrial transfer-mediated therapy
- PMID: 34586849
- PMCID: PMC8480934
- DOI: 10.1126/sciadv.abj0534
Iron oxide nanoparticles augment the intercellular mitochondrial transfer-mediated therapy
Abstract
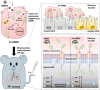
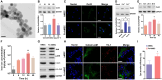
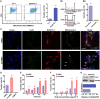
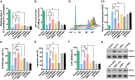
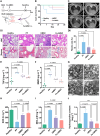
The transfer of mitochondria between cells has recently been revealed as a spontaneous way to protect the injured cells. However, the utilization of this natural transfer process for disease treatment is so far limited by its unsatisfactory transfer efficiency and selectivity. Here, we demonstrate that iron oxide nanoparticles (IONPs) can augment the intercellular mitochondrial transfer from human mesenchymal stem cells (hMSCs) selectively to diseased cells, owing to the enhanced formation of connexin 43–containing gap junctional channels triggered by ionized IONPs. In a mouse model of pulmonary fibrosis, the IONP-engineered hMSCs achieve a remarkable mitigation of fibrotic progression because of the promoted intercellular mitochondrial transfer, with no serious safety issues identified. The present study reports a potential method of using IONPs to enable hMSCs for efficient and safe transfer of mitochondria to diseased cells to restore mitochondrial bioenergetics.
Figures

References
-
- Nicolás-Ávila J. A., Lechuga-Vieco A. V., Esteban-Martinez L., Sanchez-Diaz M., Diaz-Garcia E., Santiago D. J., Rubio-Ponce A., Li J. L., Balachander A., Quintana J. A., Martínez-de-Mena R., Castejón-Vega B., Pun-Garcia A., Traves P. G., Bonzon-Kulichenko E., Garcia-Marques F., Cussó L., A-González-Guerra N., Roche-Molina M., Martin-Salamanca S., Crainiciuc G., Guzman G., Larrazabal J., Herrero-Galan E., Alegre-Cebollada J., Lemke G., Rothlin C. V., Jimenez-Borreguero L. J., Reyes G., Castrillo A., Desco M., Muñoz-Cánoves P., Ibáñez B., Torres M., Ng L. G., Priori S. G., Bueno H., Vázquez J., Cordero M. D., Bernal J. A., Enriquez J. A., Hidalgo A., A network of macrophages supports mitochondrial homeostasis in the heart. Cell 183, 94–109.e23 (2020). - PubMed
-
- Fang E. F., Hou Y., Palikaras K., Adriaanse B. A., Kerr J. S., Yang B., Lautrup S., Hasan-Olive M. M., Caponio D., Dan X., Rocktaschel P., Croteau D. L., Akbari M., Greig N. H., Fladby T., Nilsen H., Cader M. Z., Mattson M. P., Tavernarakis N., Bohr V. A., Mitophagy inhibits amyloid-β and tau pathology and reverses cognitive deficits in models of Alzheimer’s disease. Nat. Neurosci. 22, 401–412 (2019). - PMC - PubMed
LinkOut - more resources
Full Text Sources
Miscellaneous

