Visual speech differentially modulates beta, theta, and high gamma bands in auditory cortex
- PMID: 34587350
- PMCID: PMC8630510
- DOI: 10.1111/ejn.15482
Visual speech differentially modulates beta, theta, and high gamma bands in auditory cortex
Abstract
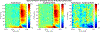
Speech perception is a central component of social communication. Although principally an auditory process, accurate speech perception in everyday settings is supported by meaningful information extracted from visual cues. Visual speech modulates activity in cortical areas subserving auditory speech perception including the superior temporal gyrus (STG). However, it is unknown whether visual modulation of auditory processing is a unitary phenomenon or, rather, consists of multiple functionally distinct processes. To explore this question, we examined neural responses to audiovisual speech measured from intracranially implanted electrodes in 21 patients with epilepsy. We found that visual speech modulated auditory processes in the STG in multiple ways, eliciting temporally and spatially distinct patterns of activity that differed across frequency bands. In the theta band, visual speech suppressed the auditory response from before auditory speech onset to after auditory speech onset (-93 to 500 ms) most strongly in the posterior STG. In the beta band, suppression was seen in the anterior STG from -311 to -195 ms before auditory speech onset and in the middle STG from -195 to 235 ms after speech onset. In high gamma, visual speech enhanced the auditory response from -45 to 24 ms only in the posterior STG. We interpret the visual-induced changes prior to speech onset as reflecting crossmodal prediction of speech signals. In contrast, modulations after sound onset may reflect a decrease in sustained feedforward auditory activity. These results are consistent with models that posit multiple distinct mechanisms supporting audiovisual speech perception.
Keywords: ECoG; audiovisual; iEEG; intracranial; multisensory; sEEG; speech.
© 2021 Federation of European Neuroscience Societies and John Wiley & Sons Ltd.
Conflict of interest statement
Conflict of Interest Statement
The authors declare no competing financial interests.
Figures




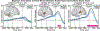
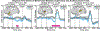
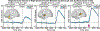

References
-
- Aarts E, Verhage M, Veenvliet JV, Dolan CV, and Van Der Sluis S (2014). A solution to dependency: using multilevel analysis to accommodate nested data. Nature neuroscience, 17(4), 491–496. - PubMed
-
- Arnal LH, Wyart V, and Giraud AL (2011). Transitions in neural oscillations reflect prediction errors generated in audiovisual speech. Nature neuroscience, 14(6), 797. - PubMed

