Evidence for constancy in the modularity of trunk muscle activity preceding reaching: implications for the role of preparatory postural activity
- PMID: 34587462
- PMCID: PMC8782652
- DOI: 10.1152/jn.00093.2021
Evidence for constancy in the modularity of trunk muscle activity preceding reaching: implications for the role of preparatory postural activity
Abstract
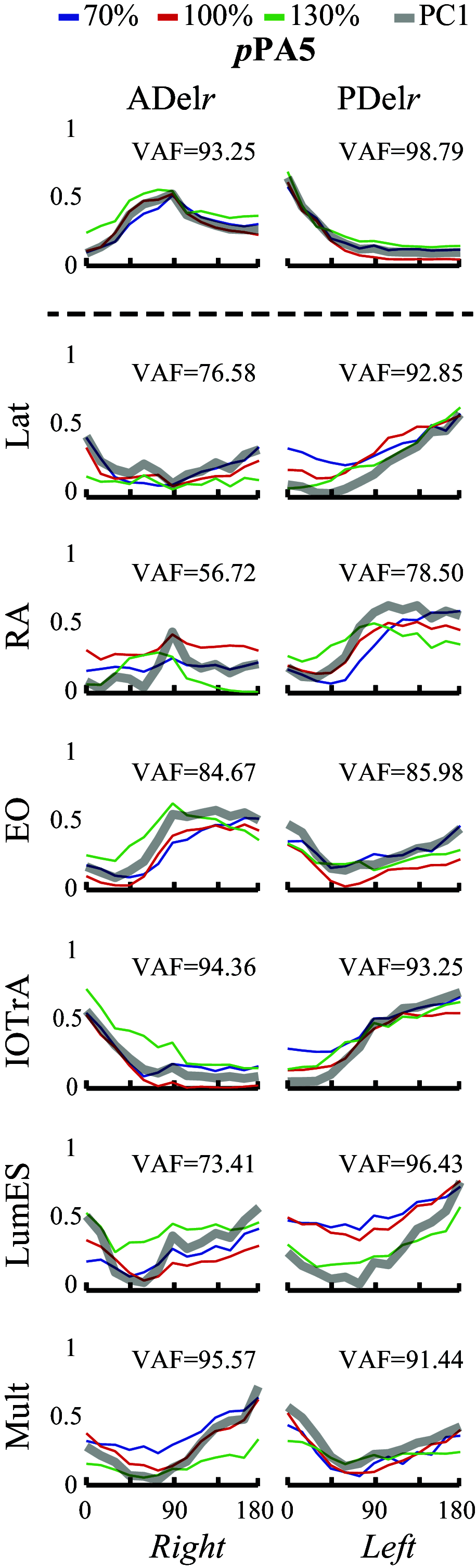
Postural muscle activity precedes voluntary movements of the upper limbs. The traditional view of this activity is that it anticipates perturbations to balance caused by the movement of a limb. However, findings from reach-based paradigms have shown that postural adjustments can initiate center of mass displacement for mobility rather than minimize its displacement for stability. Within this context, altering reaching distance beyond the base of support would place increasing constraints on equilibrium during stance. If the underlying composition of anticipatory postural activity is linked to stability, coordination between muscles (i.e., motor modules) may evolve differently as equilibrium constraints increase. We analyzed the composition of motor modules in functional trunk muscles as participants performed multidirectional reaching movements to targets within and beyond the arm's length. Bilateral trunk and reaching arm muscle activity were recorded. Despite different trunk requirements necessary for successful movement, and the changing biomechanical (i.e., postural) constraints that accompany alterations in reach distance, nonnegative matrix factorization identified functional motor modules derived from preparatory trunk muscle activity that shared common features. Relative similarity in modular weightings (i.e., composition) and spatial activation profiles that reflect movement goals across tasks necessitating differing levels of trunk involvement provides evidence that preparatory postural adjustments are linked to the same task priorities (i.e., movement generation rather than stability).NEW & NOTEWORTHY Reaching within and beyond arm's length places different task constraints upon the required trunk motion necessary for successful movement execution. The identification of constant modular features, including functional muscle weightings and spatial tuning, lend support to the notion that preparatory postural adjustments of the trunk are tied to the same task priorities driving mobility, regardless of the future postural constraints.
Keywords: coordination; motor module/synergy; postural adjustment; reach; trunk muscles.
Conflict of interest statement
No conflicts of interest, financial or otherwise, are declared by the authors.
Figures




Similar articles
-
Constancy of Preparatory Postural Adjustments for Reaching to Virtual Targets across Different Postural Configurations.Neuroscience. 2021 Feb 10;455:223-239. doi: 10.1016/j.neuroscience.2020.11.009. Epub 2020 Nov 25. Neuroscience. 2021. PMID: 33246066
-
Rapid and flexible whole body postural responses are evoked from perturbations to the upper limb during goal-directed reaching.J Neurophysiol. 2017 Mar 1;117(3):1070-1083. doi: 10.1152/jn.01004.2015. Epub 2016 Dec 21. J Neurophysiol. 2017. PMID: 28003415 Free PMC article.
-
Anticipatory weight shift between arms when reaching from a crouched posture.J Neurophysiol. 2021 Oct 1;126(4):1361-1374. doi: 10.1152/jn.00644.2020. Epub 2021 Sep 15. J Neurophysiol. 2021. PMID: 34525322
-
[From posture to initiation of movement].Rev Neurol (Paris). 1990;146(10):536-42. Rev Neurol (Paris). 1990. PMID: 2263815 Review. French.
-
Control of voluntary trunk movements in man. Mechanisms for postural equilibrium during standing.Acta Physiol Scand Suppl. 1990;595:1-60. Acta Physiol Scand Suppl. 1990. PMID: 2080712 Review.
Cited by
-
Direction-Specific Changes in Trunk Muscle Synergies in Individuals With Extension-Related Low Back Pain.Cureus. 2024 Feb 21;16(2):e54649. doi: 10.7759/cureus.54649. eCollection 2024 Feb. Cureus. 2024. PMID: 38523944 Free PMC article.
-
Variability of trunk muscle synergies underlying the multidirectional movements and stability trunk motor tasks in healthy individuals.Sci Rep. 2023 Jan 21;13(1):1193. doi: 10.1038/s41598-023-28467-6. Sci Rep. 2023. PMID: 36681745 Free PMC article.
-
Muscle synergy patterns as altered coordination strategies in individuals with chronic low back pain: a cross-sectional study.J Neuroeng Rehabil. 2023 May 31;20(1):69. doi: 10.1186/s12984-023-01190-z. J Neuroeng Rehabil. 2023. PMID: 37259142 Free PMC article.
References
-
- Bouisset S, Zattara M. A sequence of postural movements precedes voluntary movement. Neurosci Lett 22: 263–270, 1981. doi:10.1016/0304-3940(81)90117-8. - DOI
MeSH terms
Substances
Grants and funding
LinkOut - more resources
Full Text Sources

