A new extinct species of alligator lizard (Squamata: Elgaria) and an expanded perspective on the osteology and phylogeny of Gerrhonotinae
- PMID: 34587907
- PMCID: PMC8482661
- DOI: 10.1186/s12862-021-01912-8
A new extinct species of alligator lizard (Squamata: Elgaria) and an expanded perspective on the osteology and phylogeny of Gerrhonotinae
Abstract
Background: Alligator lizards (Gerrhonotinae) are a well-known group of extant North American lizard. Although many fossils were previously referred to Gerrhonotinae, most of those fossils are isolated and fragmentary cranial elements that could not be placed in a precise phylogenetic context, and only a handful of known fossils are articulated skulls. The fossil record has provided limited information on the biogeography and phylogeny of Gerrhonotinae.
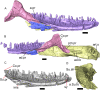
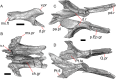
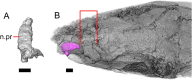
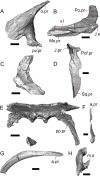
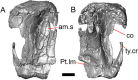
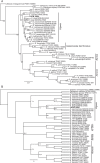
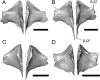
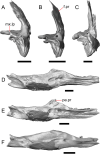
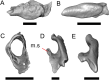
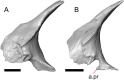
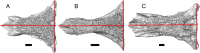
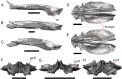
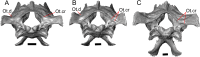
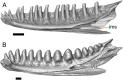
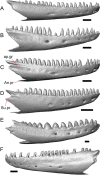
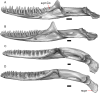
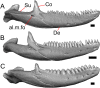
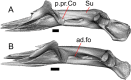
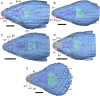
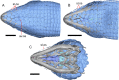
Results: We redescribe a nearly complete articulated fossil skull from the Pliocene sediments of the Anza-Borrego Desert in southern California, and refer the specimen to the alligator lizard genus Elgaria. The fossil is a representative of a newly described species, Elgaria peludoverde. We created a morphological matrix to assess the phylogeny of alligator lizards and facilitate identifications of fossil gerrhonotines. The matrix contains a considerably expanded taxonomic sample relative to previous morphological studies of gerrhonotines, and we sampled two specimens for many species to partially account for intraspecific variation. Specimen-based phylogenetic analyses of our dataset using Bayesian inference and parsimony inferred that Elgaria peludoverde is part of crown Elgaria. The new species is potentially related to the extant species Elgaria kingii and Elgaria paucicarinata, but that relationship was not strongly supported, probably because of extensive variation among Elgaria. We explored several alternative biogeographic scenarios implied by the geographic and temporal occurrence of the new species and its potential phylogenetic placements.
Conclusions: Elgaria peludoverde is the first described extinct species of Elgaria and provides new information on the biogeographic history and diversification of Elgaria. Our research expands the understanding of phylogenetic relationships and biogeography of alligator lizards and strengthens the foundation of future investigations. The osteological data and phylogenetic matrix that we provided will be critical for future efforts to place fossil gerrhonotines. Despite limited intraspecific sampled sizes, we encountered substantial variation among gerrhonotines, demonstrating the value of exploring patterns of variation for morphological phylogenetics and for the phylogenetic placement of fossils. Future osteological investigations on the species we examined and on species we did not examine will continue to augment our knowledge of patterns of variation in alligator lizards and aid in phylogenetics and fossil placement.
Keywords: Anza-Borrego; Biogeography; Elgaria; Fossils; Gerrhonotinae; Morphology; Osteology; Phylogenetics; Pliocene.
© 2021. The Author(s).
Conflict of interest statement
The authors declare that they have no competing interests.
Figures


















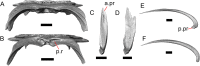








References
-
- Estes R. A new gerrhonotine lizard from the Pliocene of California. Copeia. 1963;1963:676–680. doi: 10.2307/1440971. - DOI
-
- Uetz P, Freed P, Hošek J (editors). The reptile database. http://www.reptile-database.org. 2020. Accessed 29 Apr 2021.
-
- Wilson RL. Systematics and faunal analysis of a lower Pliocene vertebrate assemblage from Trego County, Kansas. Contrib Mus Paleontol Univ Mich. 1968;22:75–126.
-
- Good DA. Phylogenetic relationships among gerrhonotine lizards: an analysis of external morphology. Univ Calif Publ Zool. 1988;121:1–139.
Publication types
MeSH terms
LinkOut - more resources
Full Text Sources
