The effect of choline-stabilized orthosilicic acid in patients with peri-implantitis: an exploratory randomized, double-blind, placebo controlled study
- PMID: 34587941
- PMCID: PMC8480141
- DOI: 10.1186/s12903-021-01817-4
The effect of choline-stabilized orthosilicic acid in patients with peri-implantitis: an exploratory randomized, double-blind, placebo controlled study
Abstract
Background: Choline-stabilized orthosilicic acid (CS-OSA) was previously found to stimulate bone collagen formation in osteopenia and to improve biomarkers of cartilage degradation in knee osteoarthritis. The aim of the present study was to investigate the effect of oral administration of CS-OSA on clinical symptoms of peri-implantitis and the associated bone loss.
Methods: Twenty-one patients with peri-implantitis were randomized in CS-OSA or placebo groups. After initial clinical and cone beam computed tomography (CBCT) measurements [probing pocket depth (PPD), bleeding on probing (BOP), mucosal recession (REC), distance from implant shoulder to alveolar crest (IS-AC) and distance from implant shoulder to first bone-to-implant contact (IS-BIC)], flap operations were performed at the peri-implantitis sites. All patients were instructed to use either placebo or CS-OSA capsules twice a day for 1 year. Measurements were repeated 6 and 12 months after randomization.
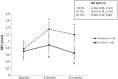
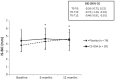
Results: The data of 18 patients (36 implants) were used in the per protocol analysis. PPD and BOP improved significantly (p < 0.05) compared to baseline for both groups after 6 and 12 months. However, REC significantly increased in the placebo group but not in the CS-OSA group. The change in REC over 6 and 12 months was significantly different between groups (p < 0.01). IS-BIC and IS-AC measurements remained stable in the CS-OSA group whereas in the placebo group, both parameters increased significantly after 6 and 12 months. The change in IS-BIC over 12 months was significantly different between groups (p < 0.05).
Conclusion: The results of this preliminary study suggest that CS-OSA may stabilize and even prevent further bone loss after surgical peri-implantitis treatment and support mucosal tissue healing. Trial registration The trial was retrospectively registered at ISRCTN registry, registration number: ISRCTN14348802, registration date: 24/06/2020.
Keywords: Bone; Choline-stabilized orthosilicic acid; Mucosal recession; Peri-implantitis.
© 2021. The Author(s).
Conflict of interest statement
SMP and IDC are employed as research associates of the study sponsor Bio Minerals NV (Belgium, the manufacturer of choline-stabilized orthosilicic acid). MCH has received financial support from Bio Minerals NV to conduct the study. WT, GUC and MT report no conflicts of interest related to this study.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

