Guanidinylated Apolipoprotein C3 (ApoC3) Associates with Kidney and Vascular Injury
- PMID: 34588185
- PMCID: PMC8638400
- DOI: 10.1681/ASN.2021040503
Guanidinylated Apolipoprotein C3 (ApoC3) Associates with Kidney and Vascular Injury
Abstract
Background: Coexistent CKD and cardiovascular diseases are highly prevalent in Western populations and account for substantial mortality. We recently found that apolipoprotein C-3 (ApoC3), a major constituent of triglyceride-rich lipoproteins, induces sterile systemic inflammation by activating the NOD-like receptor protein-3 (NLRP3) inflammasome in human monocytes via an alternative pathway.
Methods: To identify posttranslational modifications of ApoC3 in patients with CKD, we used mass spectrometry to analyze ApoC3 from such patients and from healthy individuals. We determined the effects of posttranslationally modified ApoC3 on monocyte inflammatory response in vitro, as well as in humanized mice subjected to unilateral ureter ligation (a kidney fibrosis model) and in a humanized mouse model for vascular injury and regeneration. Finally, we conducted a prospective observational trial of 543 patients with CKD to explore the association of posttranslationally modified ApoC3 with renal and cardiovascular events in such patients.
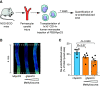
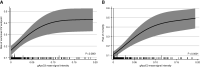
Results: We identified significant posttranslational guanidinylation of ApoC3 (gApoC3) in patients with CKD. We also found that mechanistically, guanidine and urea induce guanidinylation of ApoC3. A 2D-proteomic analysis revealed that gApoC3 accumulated in kidneys and plasma in a CKD mouse model (mice fed an adenine-rich diet). In addition, gApoC3 augmented the proinflammatory effects of ApoC3 in monocytes in vitro . In humanized mice, gApoC3 promoted kidney tissue fibrosis and impeded vascular regeneration. In CKD patients, higher gApoC3 plasma levels (as determined by mass spectrometry) were associated with increased mortality as well as with renal and cardiovascular events.
Conclusions: Guanidinylation of ApoC3 represents a novel pathogenic mechanism in CKD and CKD-associated vascular injury, pointing to gApoC3 as a potential therapeutic target.
Copyright © 2021 by the American Society of Nephrology.
Figures







References
-
- Matsushita K, van der Velde M, Astor BC, Woodward M, Levey AS, de Jong PE, et al. ; Chronic Kidney Disease Prognosis Consortium : Association of estimated glomerular filtration rate and albuminuria with all-cause and cardiovascular mortality in general population cohorts: a collaborative meta-analysis. Lancet 375: 2073–2081, 2010 - PMC - PubMed
-
- Ortiz A, Covic A, Fliser D, Fouque D, Goldsmith D, Kanbay M, et al. ; Board of the EURECA-m Working Group of ERA-EDTA : Epidemiology, contributors to, and clinical trials of mortality risk in chronic kidney failure. Lancet 383: 1831–1843, 2014 - PubMed
-
- GBD 2017 Disease and Injury Incidence and Prevalence Collaborators : Global, regional, and national incidence, prevalence, and years lived with disability for 354 diseases and injuries for 195 countries and territories, 1990–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet 392: 1789–1858, 2018 - PMC - PubMed
-
- Speer T, Zewinger S, Fliser D: Uraemic dyslipidaemia revisited: role of high-density lipoprotein. Nephrol Dial Transplant 28: 2456–2463, 2013 - PubMed
-
- Speer T, Ridker PM, von Eckardstein A, Schunk SJ, Fliser D: Lipoproteins in chronic kidney disease: from bench to bedside. Eur Heart J 42: 2170–2185, 2021 - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Miscellaneous

