R othia mucilaginosa is an anti-inflammatory bacterium in the respiratory tract of patients with chronic lung disease
- PMID: 34588194
- PMCID: PMC9068977
- DOI: 10.1183/13993003.01293-2021
R othia mucilaginosa is an anti-inflammatory bacterium in the respiratory tract of patients with chronic lung disease
Abstract
Background: Chronic airway inflammation is the main driver of pathogenesis in respiratory diseases such as severe asthma, chronic obstructive pulmonary disease, cystic fibrosis (CF) and bronchiectasis. While the role of common pathogens in airway inflammation is widely recognised, the influence of other microbiota members is still poorly understood.
Methods: We hypothesised that the lung microbiota contains bacteria with immunomodulatory activity which modulate net levels of immune activation by key respiratory pathogens. Therefore, we assessed the immunomodulatory effect of several members of the lung microbiota frequently reported as present in CF lower respiratory tract samples.
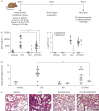
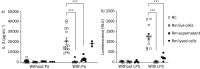
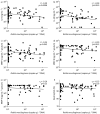
Results: We show that Rothia mucilaginosa, a common resident of the oral cavity that is also often detectable in the lower airways in chronic disease, has an inhibitory effect on pathogen- or lipopolysaccharide-induced pro-inflammatory responses, in vitro (three-dimensional cell culture model) and in vivo (mouse model). Furthermore, in a cohort of adults with bronchiectasis, the abundance of Rothia species was negatively correlated with pro-inflammatory markers (interleukin (IL)-8 and IL-1β) and matrix metalloproteinase (MMP)-1, MMP-8 and MMP-9 in sputum. Mechanistic studies revealed that R. mucilaginosa inhibits NF-κB pathway activation by reducing the phosphorylation of IκBα and consequently the expression of NF-κB target genes.
Conclusions: These findings indicate that the presence of R. mucilaginosa in the lower airways potentially mitigates inflammation, which could in turn influence the severity and progression of chronic respiratory disorders.
Copyright ©The authors 2022.
Conflict of interest statement
Conflict of interest: C. Rigauts declares a patent application related to this work. Conflict of interest: J. Aizawa has nothing to disclose. Conflict of interest: S.L. Taylor has nothing to disclose. Conflict of interest: G.B. Rogers has nothing to disclose. Conflict of interest: M. Govaerts has nothing to disclose. Conflict of interest: P. Cos has nothing to disclose. Conflict of interest: L. Ostyn has nothing to disclose. Conflict of interest: S. Sims has nothing to disclose. Conflict of interest: E. Vandeplassche has nothing to disclose. Conflict of interest: M. Sze has nothing to disclose. Conflict of interest: Y. Dondelinger has nothing to disclose. Conflict of interest: L. Vereecke has nothing to disclose. Conflict of interest: H. Van Acker has nothing to disclose. Conflict of interest: J.L. Simpson has nothing to disclose. Conflict of interest: L. Burr has nothing to disclose. Conflict of interest: A. Willems has nothing to disclose. Conflict of interest: M.M. Tunney has nothing to disclose. Conflict of interest: C. Cigana has nothing to disclose. Conflict of interest: A. Bragonzi has nothing to disclose. Conflict of interest: T. Coenye declares a patent application related to this work. Conflict of interest: A. Crabbé declares a patent application related to this work.
Figures



Comment in
-
The respiratory tract microbiome: moving from correlation to causation.Eur Respir J. 2022 May 5;59(5):2103079. doi: 10.1183/13993003.03079-2021. Print 2022 May. Eur Respir J. 2022. PMID: 35512808 No abstract available.
References
-
- Tsikrika S, Dimakou K, Papaioannou A, et al. . Non-invasive evaluation of airway inflammation in non-CF bronchiectasis. Eur Respir J 2016; 48: Suppl. 60, PA2556. doi:10.1183/13993003.congress-2016.PA2556 - DOI
-
- Ishmael FT. The inflammatory response in the pathogenesis of asthma. J Am Osteopath Assoc 2011; 111: 11 Suppl. 7, S11–S17. - PubMed
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical
Molecular Biology Databases
Miscellaneous
