Open-label, randomised, clinical trial to evaluate the immunogenicity and safety of a prophylactic vaccination of healthcare providers by administration of a heterologous vaccine regimen against Ebola in the Democratic Republic of the Congo: the study protocol
- PMID: 34588237
- PMCID: PMC8479954
- DOI: 10.1136/bmjopen-2020-046835
Open-label, randomised, clinical trial to evaluate the immunogenicity and safety of a prophylactic vaccination of healthcare providers by administration of a heterologous vaccine regimen against Ebola in the Democratic Republic of the Congo: the study protocol
Abstract
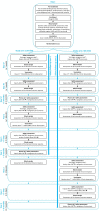
Introduction: This article describes the protocol of an Ebola vaccine clinical trial which investigates the safety and immunogenicity of a two-dose prophylactic Ebola vaccine regimen comprised of two Ebola vaccines (Ad26.ZEBOV and MVA-BN-Filo) administered 56 days apart, followed by a booster vaccination with Ad26.ZEBOV offered at either 1 year or 2 years (randomisation 1:1) after the first dose. This clinical trial is part of the EBOVAC3 project (an Innovative Medicines Initiative 2 Joint Undertaking), and is the first to evaluate the safety and immunogenicity of two different booster vaccination arms in a large cohort of adults.
Methods and analysis: This study is an open-label, monocentric, phase 2, randomised vaccine trial. A total of 700 healthcare providers and frontliners are planned to be recruited from the Tshuapa province in the Democratic Republic of the Congo (DRC). The primary and secondary objectives of the study assess the immunogenicity of the first (Ad26.ZEBOV), second (MVA-BN-Filo) and booster (Ad26.ZEBOV) dose. Immunogenicity is assessed through the evaluation of EBOV glycoprotein binding antibody responses after vaccination. Safety is assessed through the collection of serious adverse events from the first dose until 6 months post booster vaccination and the collection of solicited and unsolicited adverse events for 1 week after the booster dose.
Ethics and dissemination: The protocol was approved by the National Ethics Committee of the Ministry of Health of the DRC (n°121/CNES/BN/PMMF/2019). The clinical trial was registered on 4 December 2019 on ClinicalTrials.gov. Trial activities are planned to finish in October 2022. All participants are required to provide written informed consent and no study-related procedures will be performed until consent is obtained. The results of the trial will be added on ClinicalTrials.gov, published in peer-reviewed journals and presented at international conferences.
Trial registration number: NCT04186000; Pre-results.
Keywords: health & safety; immunology; protocols & guidelines.
© Author(s) (or their employer(s)) 2021. Re-use permitted under CC BY-NC. No commercial re-use. See rights and permissions. Published by BMJ.
Conflict of interest statement
Competing interests: None declared.
Figures
References
-
- World Health Organization . Ebola virus disease. Fact sheet N 103, 2014.
Publication types
MeSH terms
Substances
Associated data
LinkOut - more resources
Full Text Sources
Medical
