Effects of hypoxia and nanocarrier size on pH-responsive nano-delivery system to solid tumors
- PMID: 34588504
- PMCID: PMC8481507
- DOI: 10.1038/s41598-021-98638-w
Effects of hypoxia and nanocarrier size on pH-responsive nano-delivery system to solid tumors
Abstract
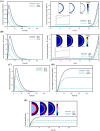
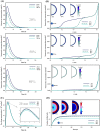
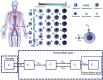
One of the special features of solid tumors is the acidity of the tumor microenvironment, which is mainly due to the presence of hypoxic regions. Therefore, pH-responsive drug delivery systems have recently been highly welcomed. In the present study, a comprehensive mathematical model is presented based on extravascular drug release paradigm. Accordingly, drug delivery system using pH-responsive nanocarriers is taken into account to examine the impacts of hypoxic regions as well as the size of nanocarriers for cancerous cell-death. The extent of hypoxic regions is controlled by vascular density. This means that regions with very low vascular density represent regions of hypoxia. Using this mathematical model, it is possible to simulate the extracellular and intracellular concentrations of drug by considering the association/disassociation of the free drug to the cell-surface receptors and cellular uptake. Results show that nanocarriers with smaller sizes are more effective due to higher accumulation in the tumor tissue interstitium. The small size of the nanocarriers also allows them to penetrate deeper, so they can expose a larger portion of the tumor to the drug. Additionally, the presence of hypoxic regions in tumor reduces the fraction of killed cancer cells due to reduced penetration depth. The proposed model can be considered for optimizing and developing pH-sensitive delivery systems to reduce both cost and time of the process.
© 2021. The Author(s).
Conflict of interest statement
The authors declare no competing interests.
Figures



References
-
- Folkman J. How is blood vessel growth regulated in normal and neoplastic tissue? GHA Clowes Mem. Award Lecture Cancer Res. 1986;46:467–473. - PubMed
MeSH terms
Substances
LinkOut - more resources
Full Text Sources
Medical

