The biochemical subtype is a predictor for cognitive function in glutaric aciduria type 1: a national prospective follow-up study
- PMID: 34588557
- PMCID: PMC8481501
- DOI: 10.1038/s41598-021-98809-9
The biochemical subtype is a predictor for cognitive function in glutaric aciduria type 1: a national prospective follow-up study
Erratum in
-
Publisher Correction: The biochemical subtype is a predictor for cognitive function in glutaric aciduria type 1: a national prospective follow-up study.Sci Rep. 2021 Oct 12;11(1):20618. doi: 10.1038/s41598-021-00137-5. Sci Rep. 2021. PMID: 34642359 Free PMC article. No abstract available.
Abstract
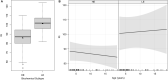
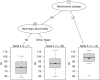
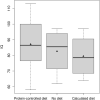
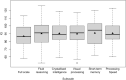
The aim of the study was a systematic evaluation of cognitive development in individuals with glutaric aciduria type 1 (GA1), a rare neurometabolic disorder, identified by newborn screening in Germany. This national, prospective, observational, multi-centre study includes 107 individuals with confirmed GA1 identified by newborn screening between 1999 and 2020 in Germany. Clinical status, development, and IQ were assessed using standardized tests. Impact of interventional and non-interventional parameters on cognitive outcome was evaluated. The majority of tested individuals (n = 72) showed stable IQ values with age (n = 56 with IQ test; median test age 11 years) but a significantly lower performance (median [IQR] IQ 87 [78-98]) than in general population, particularly in individuals with a biochemical high excreter phenotype (84 [75-96]) compared to the low excreter group (98 [92-105]; p = 0.0164). For all patients, IQ results were homogenous on subscale levels. Sex, clinical motor phenotype and quality of metabolic treatment had no impact on cognitive functions. Long-term neurologic outcome in GA1 involves both motor and cognitive functions. The biochemical high excreter phenotype is the major risk factor for cognitive impairment while cognitive functions do not appear to be impacted by current therapy and striatal damage. These findings implicate the necessity of new treatment concepts.
© 2021. The Author(s).
Conflict of interest statement
E. M. C. Märtner, E. Thimm, P. Guder, K. A. Schiergens, F. Rutsch, S. Roloff, I. Marquardt, A. M. Das, P. Freisinger, S. C. Grünert, J. Krämer, M. R. Baumgartner, S. Beblo, C. Haase, A. Dieckmann, M. Lindner, A. Näke, G. F. Hoffmann, C. Mühlhausen, M. Walter, S. F. Garbade, E. M. Maier, S. Kölker, and N. Boy declare no disclosures relevant to the manuscript.
Figures

References
-
- Boy N, Mengler K, Thimm E, et al. Newborn screening: A disease-changing intervention for glutaric aciduria type 1. Ann. Neurol. 2018;83:970–979. - PubMed
-
- Kolker S, Garbade SF, Greenberg CR, et al. Natural history, outcome, and treatment efficacy in children and adults with glutaryl-CoA dehydrogenase deficiency. Pediatr. Res. 2006;59:840–847. - PubMed
-
- Strauss KA, Puffenberger EG, Robinson DL, Morton DH. Type I glutaric aciduria, part 1: natural history of 77 patients. Am. J. Med. Genet. C Semin. Med. Genet. 2003;121C:38–52. - PubMed
-
- Kolker S, Garcia-Cazorla A, Valayannopoulos V, et al. The phenotypic spectrum of organic acidurias and urea cycle disorders. Part 1: the initial presentation. J. Inherited Metab. Dis. 2015;38:1041–1057. - PubMed
-
- Baric I, Wagner L, Feyh P, Liesert M, Buckel W, Hoffmann GF. Sensitivity and specificity of free and total glutaric acid and 3-hydroxyglutaric acid measurements by stable-isotope dilution assays for the diagnosis of glutaric aciduria type I. J. Inherit. Metab. Dis. 1999;22:867–881. - PubMed

