Efficacy of CDK4/6 inhibitors in preclinical models of malignant pleural mesothelioma
- PMID: 34588615
- PMCID: PMC8576019
- DOI: 10.1038/s41416-021-01547-y
Efficacy of CDK4/6 inhibitors in preclinical models of malignant pleural mesothelioma
Abstract
Background: There is no effective therapy for patients with malignant pleural mesothelioma (MPM) who progressed to platinum-based chemotherapy and immunotherapy.
Methods: We aimed to investigate the antitumor activity of CDK4/6 inhibitors using in vitro and in vivo preclinical models of MPM.
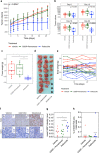
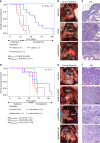
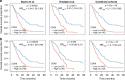
Results: Based on publicly available transcriptomic data of MPM, patients with CDK4 or CDK6 overexpression had shorter overall survival. Treatment with abemaciclib or palbociclib at 100 nM significantly decreased cell proliferation in all cell models evaluated. Both CDK4/6 inhibitors significantly induced G1 cell cycle arrest, thereby increasing cell senescence and increased the expression of interferon signalling pathway and tumour antigen presentation process in culture models of MPM. In vivo preclinical studies showed that palbociclib significantly reduced tumour growth and prolonged overall survival using distinct xenograft models of MPM implanted in athymic mice.
Conclusions: Treatment of MPM with CDK4/6 inhibitors decreased cell proliferation, mainly by promoting cell cycle arrest at G1 and by induction of cell senescence. Our preclinical studies provide evidence for evaluating CDK4/6 inhibitors in the clinic for the treatment of MPM.
© 2021. The Author(s), under exclusive licence to Springer Nature Limited.
Conflict of interest statement
EN received research support from Roche, Pfizer, Bristol Myers Squibb and Merck Serono and participated in advisory boards from Bristol Myers Squibb, Merck Serono, Merck Sharpe & Dohme, Lilly, Roche, Pfizer, Takeda, Boehringer Ingelheim, Bayer, Amgen and Astra Zeneca. RP has participated in advisory boards from Bristol Myers Squibb, Merck Sharpe & Dohme, Roche, Pfizer, Lilly, Boehringer Ingelheim and Astra Zeneca. DF has received research support from Astex Therapeutics, Astra Zeneca, Bayer, Boehringer Ingelheim, Bristol Myers Squibb, Clovis Oncology, Merck Sharpe & Dohme, Lilly Oncology, Roche and participated in advisory boards from Atara Therapeutics, Bayer, Boehringer Ingelheim and Inventiva. The other authors declare no competing interests.
Figures


References
Publication types
MeSH terms
Substances
LinkOut - more resources
Full Text Sources

