Neuroprotective effects of Bhilawanol and Anacardic acid during glutamate-induced neurotoxicity
- PMID: 34588850
- PMCID: PMC8463467
- DOI: 10.1016/j.jsps.2021.07.011
Neuroprotective effects of Bhilawanol and Anacardic acid during glutamate-induced neurotoxicity
Abstract
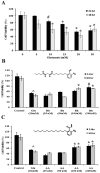
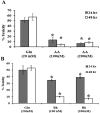
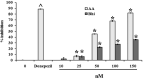
Bhilawanol (Bh) and anacardic acid (AA) are two lipid-soluble compounds mostly found in the nut of Semecarpus anacardium (SA). This herb has many medicinal properties including enhancing learning and memory, yet its active compounds have not been studied for neuroprotective effects. We investigated the neuroprotective effects of Bh and AA against glutamate induced cell death in the adrenal pheochromocytoma cell line of rats (PC12 cells). Cell viability, toxicity and calcium influx were determined by MTT assay, LDH release assay and Fluo-3 imaging while apoptosis was assayed by caspase-3 and Bcl-2 gene expression. Our results showed that Bh and AA treatments significantly increased cell viability, reduced cell toxicity and calcium influx in PC12 cells in addition to suppressing the reactive oxygen species. Furthermore, AA treatment decreased caspase-3 expression level whereas both Bh and AA enhanced the expression of anti-apoptotic gene Bcl-2 in PC12 cells. Both compounds potently inhibited acetylcholinesterase enzyme (AChE) in a dose and time dependent manner. These findings suggest that the traditional use of SA may be explained on the basis of both Bh and AA showing neuroprotective potential due to their effects on enhancing cell viability, reducing cell toxicity most probably by reducing excessive calcium influx and suppression of ROS as well as by decreasing the expression of proapoptotic caspase 3 gene and increasing the expression of antiapoptotic gene Bcl2. Traditional use in enhancing learning and memory was justified in part by inhibition of AChE.
Keywords: Anacardic acid; Bcl-2; Bhilawanol; Caspase-3; LDH assay; MTT assay; Semecarpus anacardium.
© 2021 The Author(s).
Conflict of interest statement
The authors declare that they have no known competing financial interests or personal relationships that could have appeared to influence the work reported in this paper.
Figures


References
-
- Abdullah A.-S.-H., Mohammed A.S., Abdullah R., Mirghani M.E.S., Al-Qubaisi M. Cytotoxic effects of Mangifera indica L. kernel extract on human breast cancer (MCF-7 and MDA-MB-231 cell lines) and bioactive constituents in the crude extract. BMC Complementary and Alternative Medicine. 2014;14:199. doi: 10.1186/1472-6882-14-199. - DOI - PMC - PubMed
LinkOut - more resources
Full Text Sources
Other Literature Sources
Research Materials

