Gasdermin D in pyroptosis
- PMID: 34589396
- PMCID: PMC8463274
- DOI: 10.1016/j.apsb.2021.02.006
Gasdermin D in pyroptosis
Abstract
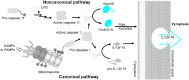
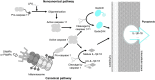
Pyroptosis is the process of inflammatory cell death. The primary function of pyroptosis is to induce strong inflammatory responses that defend the host against microbe infection. Excessive pyroptosis, however, leads to several inflammatory diseases, including sepsis and autoimmune disorders. Pyroptosis can be canonical or noncanonical. Upon microbe infection, the canonical pathway responds to pathogen-associated molecular patterns (PAMPs) and damage-associated molecular patterns (DAMPs), while the noncanonical pathway responds to intracellular lipopolysaccharides (LPS) of Gram-negative bacteria. The last step of pyroptosis requires the cleavage of gasdermin D (GsdmD) at D275 (numbering after human GSDMD) into N- and C-termini by caspase 1 in the canonical pathway and caspase 4/5/11 (caspase 4/5 in humans, caspase 11 in mice) in the noncanonical pathway. Upon cleavage, the N-terminus of GsdmD (GsdmD-N) forms a transmembrane pore that releases cytokines such as IL-1β and IL-18 and disturbs the regulation of ions and water, eventually resulting in strong inflammation and cell death. Since GsdmD is the effector of pyroptosis, promising inhibitors of GsdmD have been developed for inflammatory diseases. This review will focus on the roles of GsdmD during pyroptosis and in diseases.
Keywords: 7DG, 7-desacetoxy-6,7-dehydrogedunin; ADRA2B, α-2B adrenergic receptor; AIM, absent in melanoma; ASC, associated speck-like protein; Ac-FLTD-CMK, acetyl-FLTD-chloromethylketone; BMDM, bone marrow-derived macrophages; CARD, caspase activation; CD, Crohn’s disease; CTM, Chinese traditional medicine; CTSG, cathepsin G; Caspase; DAMP, damage-associated molecular pattern; DFNA5, deafness autosomal dominant 5; DFNB59, deafness autosomal recessive type 59; DKD, diabetic kidney disease; DMF, dimethyl fumarate; Damage-associated molecular patterns (DAMPs); ELANE, neutrophil expressed elastase; ESCRT, endosomal sorting complexes required for transport; FADD, FAS-associated death domain; FDA, U.S. Food and Drug Administration; FIIND, function to find domain; FMF, familial Mediterranean fever; GI, gastrointestinal; GPX, glutathione peroxidase; Gasdermin; GsdmA/B/C/D/E, gasdermin A/B/C/D/E; HAMP, homeostasis altering molecular pattern; HIN, hematopoietic expression, interferon-inducible nature, and nuclear localization; HIV, human immunodeficiency virus; HMGB1, high mobility group protein B1; IBD, inflammatory bowel disease; IFN, interferon; ITPR1, inositol 1,4,5-trisphosphate receptor type 1; Inflammasome; Inflammation; LPS, lipopolysaccharide; LRR, leucine-rich repeat; MAP3K7, mitogen-activated protein kinase kinase kinase 7; MCC950, N-[[(1,2,3,5,6,7-hexahydro-s-indacen-4-yl)amino]carbonyl]-4-(1-hydroxy-1-methylethyl)-2-furansulfonamide; NAIP, NLR family apoptosis inhibitory protein; NBD, nucleotide-binding domain; NEK7, NIMA-related kinase 7; NET, neutrophil extracellular trap; NIK, NF-κB inducing kinase; NLR, NOD-like receptor; NLRP, NLR family pyrin domain containing; NSAID, non-steroidal anti-inflammatory drug; NSCLC, non-small cell lung cancer; NSP, neutrophil specific serine protease; PAMP, pathogen-associated molecular pattern; PKA, protein kinase A; PKN1/2, protein kinase1/2; PKR, protein kinase-R; PRR, pattern recognition receptors; PYD, pyrin domain; Pathogen-associated molecular patterns (PAMPs); Pyroptosis; ROS, reactive oxygen species; STING, stimulator of interferon genes; Sepsis; TLR, Toll-like receptor; UC, ulcerative colitis; cAMP, cyclic adenosine monophosphate; cGAS, cyclic GMP–AMP synthase; mtDNA, mitochondrial DNA.
© 2021 Chinese Pharmaceutical Association and Institute of Materia Medica, Chinese Academy of Medical Sciences. Production and hosting by Elsevier B.V.
Conflict of interest statement
The authors declare no conflicts of interest.
Figures


References
-
- Heilig R., Dick M.S., Sborgi L., Meunier E., Hiller S., Broz P. The Gasdermin-D pore acts as a conduit for IL-1β secretion in mice. Eur J Immunol. 2018;48:584–592. - PubMed
Publication types
Grants and funding
LinkOut - more resources
Full Text Sources
Research Materials
Miscellaneous

